Images of cells observed in vivo
Speaking
of Fixation:
Part
1
by M. Halit Umar
In this article, our intention is to find an answer to a fundamental question in microscopy; it relates to a process called 'fixation'. When and why do we need to fix cells or tissues before microscopical study? And how can we achieve an optimal fixation? These questions are frequently posed.The subject of fixation and preservation is a major scientific topic in the methodology of microscopy and it deals with many aspects of the living state and the nature of the organisms to be fixed. The fixation of living cells and tissues simply means an immediate stop of the life processes taking place within and around them. Time becomes frozen for living things after fixation.
Any fixation must also be a tool for preservation for a long time, otherwise it may cause disintegration of the structures, which is of course not our aim.
Most of us, as amateur microscopists, like to study cells/tissues of various origin in their natural environment and in the living state. A drop of pond water is a miniature copy of the freshwater pool in which many organisms live. When such material is sampled and studied with a microscope, we observe living organisms in their natural environment; so we speak of in vivo and in situ microscopy. Let's dry the drop and observe it once again. No further movement occurs. The living organisms are no longer alive, they are fixed on the glass slide for ever. Their transparency is also diminished. This is an example of drying and fixing at ambient temperature. Freezing or higher temperatures physically act as a fixing agent. The heat produced by a flame, for example, is a well-known method of fixation in bacteriology.
Another manner of fixation is so-called 'chemical fixation.' Depending on the physiological status of the cells/tissues to be fixed, numerous chemical fixatives were defined in the past centuries and the techniques are still being improved. It is obvious that different types of cells/tissues can be treated by different kinds of fixatives to achieve a good preserving effect. An organism from salt water (say, the ocean) probably cannot be fixed with a solution most applicable for pond water organisms. And animal cells have no cell walls, in sharp contrast to plant cells and fungi. Any fixative for the latter has to cross through the wall to fix cell structures, the organelles, behind it.
Of the many chemical fixatives still in use, none of them are perfect but an approximation to an optimal condition for preserving. The following images will demonstrate to us that the same material, in this case algae collected from pond water, reacts in surprisingly different ways after fixation. I used a mixture of formaldehyde and glutaraldehyde in phosphate buffer. This everyday fixative is used for both light- and electron microscopy and its effect has been proved as optimal for animal, plant and fungal cells. As far as I can search in English references, it is not applied yet on the freshwater organisms as illustrated below.
One benefit of fixation, is that it allows the material to be embedded in various kinds of matrices like paraffin or plastic resin, and cut as thin sections. Most of the images that I present below are obtained from a material embedded in GMA (glycol methacrylate; a plastic matrix for embedding) and cut 3 micrometres thick.
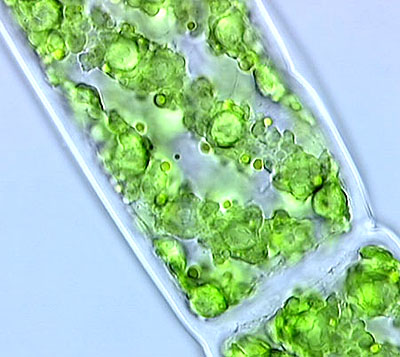
The first three images illustrate features of freshly collected Spirogyra (a filamentous alga) as seen by light microscopy. No fixation or staining has been performed prior to microscopy; it is therefore an in vivo study since the life processes of these organisms continue for a while until water evaporates and the material consequently dries. We currently accept that life exists only in the presence of water. Extracting water from tissues and cells is a process called 'dehydration'. Dehydration can be achieved by techniques like heating or by using dehyrating agents like alcohols. Dehydration causes the molecular machinery inside the cells to stop and irreversible changes occur in the molecular structures comprising various cell components, including cell membranes and walls. The ultimate effect of dehydration is cell death by an increase in cell membrane permeability.
Images of cells observed in vivo

Fig. 1. Note the almost transparent cell wall and septum between two segments. The chloroplasts are arranged in a spiral pattern. For the ultrastructural details of the chloroplasts refer to Speaking of Cells in the Sept. 2000 Micscape..
Fig. 2. The septum physically divides spirogyral cells which form long filaments.
.
Fig. 3. On the left side of the septum we find some cytoplasmic variations that may be signs of some kinds of cellular secretion. Remember that cell walls when newly-formed must seal off the cell from the outside. Note the existence of an almost transparent zone just beyond the cell walls. This is a very important site of the interaction between spirogyra and its micro-environment. Some algae like Ulothrix produce large amounts of slimy substances which may be observable if specific measures are taken.
.
Fig. 4. Ulothrix sp. stained in vivo with a very dilute (.001%) aqueous solution of Acridine Orange by adding just a tiny drop of the stain into the drop of pond water containing these organisms. Note that the mucous (slimy) substance around the thread-like structure of the Ulothrix. We call this dynamic substance the extracellular matrix (ECM). Although ECM is dominantly amorphous and frequently transparent, it is very rich in sugar and protein molecules and aids physical/chemical communication between filaments and cells in all organisms either uni- or multicellular.
~~~~~
Images of material after using fixation technique
Fig. 5 illustrates material gathered from the same freshwater pond. In the plastic container of pond water an equal volume of fixative solution, which is a mixture of formaldehyde and glutaraldehyde, was added. Within a few minutes all cellular movements had ceased; the material was thus chemically fixed. A subsequent dehydration and embedding in GMA took place. Thin sections were obtained and stained with Crystal Violet and Toluidine Blue. At this low magnification we see a mixture of uni- or multicellular organisms, in various forms and stainability.
.
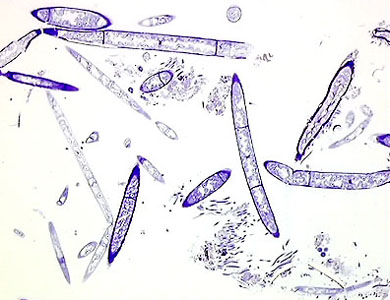
Fig. 6. A higher magnification of the same section.
.
Fig. 7. This image shows two threads of spirogyra from the same section (after fixation, dehydration, embedding, sectioning and staining with Crystal Violet). Compare this image with the first 3 images showing in vivo spirogyra from the same pond. Have you already detected the nucleus in the middle of the cells shown as a large pink mass with a centrally positioned nucleolus (the large, darkly blue stained sphere). All chloroplasts are slightly pale and with a degraded structure if you compare them with those seen in Figs 1-3.
.
Fig. 8. A drop of pond water also contains various species of animals from microscopical to sub-macroscopical size. They contrast easily with their relatively larger cells that may be stained very differently and, in fact, colourfully.
.
Fig. 9. When material is properly fixed, some details become more clear to us, especially after embedding, sectioning and staining. Note that the spirogyra filament shown here is swollen, and also degenerating due to attack of parasitizing microorganisms.
.
Fig. 10. Compare these two spirogyra filaments which are in decline and have diminished cellular and structural complexity.The organelles seem shapeless ghosts inside them. This is a microscopical feature of ageing and not an artifact of the fixation.
.
Fig. 11 above & Fig. 12 below are added to illustrate that when the material (cells and tissues) are fixed, various microscopical techniques can be applied such as embedding, sectioning and staining. By fixing such a community of cells and organisms, we create a frozen image illustrating a very typical moment in their interrelationship and interaction.
.
Fig. 12. We see here a recently completed division of a cell and a septum being formed. The nuclei are prominent, a few chloroplasts and cytoplasm are visible. Such fine detail may be indistinct if we look at it in vivo and not fixed.
Reference : The Micscape article Speaking of Cells may be used as a general illustrated introduction to cytology.Some Interesting Links :
Buffers & Chemicals in Histology: http://www.emsdiasum.com/ems/chemicals/buffers.html
Preparing Smears & Simple Staining: http://www.gpc.peachnet.edu/~ddonald/biolab/smearstain.htm
Almost everything about Microscopy & Staining: http://www.phage.org/black03.htm
Comments to the author M. Halit Umar are welcomed.
~~~~ The author's affiliation:
MES Laboratories, P.O. Box: 6042,
5960 AA HORST, The Netherlands. Phone: (0031) 77 464 7575 , FAX: (0031) 77 464 1567
All images presented in this article are registered and copyright to MES Laboratories.
~~~~~~~
Please report any Web problems or
offer general comments to the Micscape
Editor,
via the contact on current Micscape
Index.
Micscape is the on-line monthly magazine
of the Microscopy UK web
site at Microscopy-UK