|
Thecamoeba: The Shar-Pei of the Protists by Richard L. Howey, Wyoming, USA |
Last summer, we had a large hanging pot of Lobelia on our patio which I watered thoroughly every day until water would come out through the holes in the bottom of the pot. At this altitude, 7,200 feet, it is essential to water such plants on a daily basis, because the summer sun is so intense that missing even one day can produce not only dehydration and wilting, but scorching of the flowers and leaves as well. I sometimes wonder how our ancestors made it through without sun screen and insect repellent. Since, for the last 5 years we have had near drought conditions, I didn’t want the extra water to go to waste and so, I had a 5 gallon plastic bucket sitting below the pot to collect the overflow. Over the course of the summer, a rich green film developed on the surface of the overflow of such splendor that no right-thinking microscopist could resist taking a sample to examine
I was expecting algae, mostly Chlorella—not exactly a mover and shaker in the protist world—but, to my amazement, I found phytoflagellates, filamentous algae, hypotrichs, rotifers of the genus Philodina, Cyclidium, and at least 3 species of amoeba, including Thecamoeba. One of my first thoughts was: Where did all this stuff come from? How did it get into my bucket? Furthermore, this bucket is one which I use to sort marine organisms, which arrive preserved in formaldehyde, from a supplier in Maine. I rinse the bucket afterwards, but there must be chemical residues. This, perhaps is, simply another example of how extraordinarily adaptable many “primitive” organisms are. However, since I don’t believe in spontaneous generation, the question remains: Where did all this stuff come from?
It’s likely that some cysts and spores were brought in by the wind, but it’s virtually impossible to determine which organism might have arrived by such transport. Chilomonas and Chlorella seem good candidates, since a dish of water with a bit of organic debris and adequate light seems almost invariably to produce these critters. If, in addition, you live in a windy area, as I do, some other types of cysts and spores in the dust might well reach my bucket. (Merchants here sell sweat shirts with the message:
Wyoming Wind Festival:
January 1 to December 31
Nonetheless, it would still be extraordinarily difficult to determine which organisms might have been carried in such a manner. The eggs of some rotifers are remarkably resistant to dessication and, relatively speaking, are nearly weightless, So this could account for the presence of Philodina in the bucket “culture.” Protozoologists long thought that they knew what Paramecia cysts looked like and, if you consult Kudo and other early major reference works, you can even find drawings of the cysts. However, now some investigators are not so sure that we really do know what Paramecium cysts look like and, to my knowledge, no one has actually observed its excystment. The ubiquity of Paramecia certainly suggests that a key survival strategy for them is encystment, but it does seem odd that it has not been directly observed.
The variety and profusion of the organisms in my bucket are, I think, attributable to a number of factors. Indeed, I think some arrived airborne, however, I suspect that the major source was from the potting soil around the Lobelia. Nature is so unpredictable—I would have expected a fair number of Colpoda which regularly show up in soil samples, but no, I get Philodina and filamentous algae. Mind you, I’m not complaining, just curious. There are two other odd little factors which might play a role in the abundance of the dominant organisms. As winter approached, I took some samples from the bucket and created six small cultures in my lab. I haven’t added any food; I just add water twice a week. Four of them are in a “clean well-lighted place” and the other two are in quite dim light. Still all kinds of things are flourishing even after 2 and ½ years. The water I used both inside and outside (for the Lobelia) was chlorinated tap water. Also, I am almost certain that there was a tiny amount of residual formaldehyde in the bucket. We all know that dissolved chlorine in tap water dissipates fairly quickly on exposure to air and is largely gone after 24 hours. However, in this case, remember that the bucket was getting a fresh dose of chlorinated water every 24 hours, albeit, filtered though the potting soil. It is also well-known that while chlorine keeps most of the nastier critters out of our drinking water, there are others, fortunately most benign, which seem largely unaffected or even thrive as a consequence of reduced competition for food and the reduction of potential parasites as well as bacterial and viral infections. Yes, even protists have parasites and can be attacked by pathogens. How right Augustus de Morgan was when he wrote:
“Great fleas have little fleas upon their backs to bite ’em,
And little fleas have lesser fleas, and so ad infinitum,
And the great fleas themselves, in turn, have greater fleas to go on,
While these again have greater still, and so on.”
This was a takeoff on Jonathan Swift’s poem:
“So, naturalists observe, a flea
Has smaller fleas that on him prey;
And these have smaller still to bite ’em;
And so proceed ad infintum”
And a more recent poet also had a comment on fleas creating what is claimed to be the shortest poem ever written.
Fleas
“Adam
Had ’em”
Ogden Nash
There are even protists that parasitize other protists. One of the most intriguing of many examples is a small orange suctorian which lives at the posterior end of the large green (from feeding on filamentous algae) ciliate Nassula ornata—and feeds on its protoplasm. Sometimes several individuals can be observed attached to Nassula. To my knowledge, no one has studied the degree of damage which this suctorian, Podophrya parastica, inflicts to determine whether it’s one of those clever critters that “merely” feeds on and weakens its host or if it’s one of those deadly parasites that eventually kills its host. Protists can also be attacked by bacteria and viruses and sometimes when ingested they turn the tables and find a niche within the protist wherein they can flourish, sometimes innocuously and sometimes to the detriment of the host.
When I was trying to develop a protocol for culturing Lacrymaria, on several occasions, just as I thought I was making some significant progress, all of my Lacrymaria would die off within a few days, even though they were in a variety of dishes containing different media. A colleague and I finally came to the conclusion that the cultures were somehow getting hit by a viral infection. I decided to start adding a few drops of a very dilute solution of highly toxic stain to each new culture. I tried a few drops of Acridine Orange ranging in concentration from 0.01% to 0.001%. Acridine Orange is a fascinating substance as it is one of the most useful general fluorchromes for fluorescence microscopy, but must be handled with great CAUTION: it is poisonous, carcinogenic, mutagenic, and photoactive. In one culture, I ended up with some
3-headed Lacrymaria and from time to time I have tried to duplicate these results without success. It was an early experiment and I didn’t keep careful enough notes on the concentration. If I want to get a quick look at nuclei, I sometimes put a drop of culture on a slide and add a drop of 0.01% Acridine Orange at the edge of the cover glass. When I expose them to the fluorescence of the light source, they die very quickly from that concentration, since the light activates the pigment making it lethal, but the nuclei stand out strikingly.
With low levels of Acridine Orange, the Lacrymaria not only survive, but thrive; in fact, the reproductive rate seemed to increase. There is a fascinating phenomenon known as hormesis, which is a description of a situation wherein a substance, ordinarily toxic and dangerous to an organism, becomes beneficial in very, very small quantities. In the case of my Lacrymaria cultures, I suspect that the viruses were either killed or rendered non-infectious. Walter Dioni recently published a provocative article on Micscape regarding the use of highly diluted formaldehyde as a relaxant for a variety of aquatic organisms, including some protists. I decided to try out this relaxant on a few Lacrymaria. I placed about 10 ml. of culture fluid in a small Petri dish and added a few Lacrymaria and a drop of the formaldehyde relaxant. This results in a weaker solution than Walter recommends, but I had already tried the recommended concentration on a slide with fatal results. As he points out in his article, medium to large ciliates are frequently hypersensitive to chemicals introduced into their environments and, as a consequence, for over 2 centuries microscopists have been searching for effective means to relax or anesthetize ciliates in order to study them more closely in a living, albeit, altered state. Over the last 3 weeks, I have added a large drop of the formaldehyde relaxant to the Petri dish every 2 or 3 days and am now up to a total 9 drops. The number of Lacrymaria has increased, they are very active and thin and demonstrate the remarkable flexibility and extensility of the “neck”. There are virtually no other large organisms in the sample and the very small ciliates and flagellates remaining as food organisms seem to be ample, but are not overabundant. All of these factors combined suggest to me hormesis, that is, the formaldehyde relaxant, has proved to be beneficial, at least, in the short period I have been using it. I will continue to monitor it for several more week and if good results continue, I will initiate some further experiments.
No doubt you are now wondering: what the devil does all of this have to do with Thecamoeba? Well, remember the formaldehyde residue and the chlorinated tap water? What I suspect, but have no way of proving at the moment, since my “bucket culture” on the patio is at present a big cylinder of ice—it snowed here today—is that the traces of formaldehyde and chlorine had an interesting selective effect. As I further examined the samples which I established as cultures in my lab before the winter set in, I found a surprising variety of amoeba; some hardy hypotrichs and rotifers, both of which are highly adaptable and a variety of algae and flagellates. However, no Colpoda, Chilomonas, or Paramecium-all likely candidates for such cultures. So perhaps, just perhaps, this “bucket culture” may in part owe its interesting variety of algae and amoebae to the formaldehyde and chlorine.
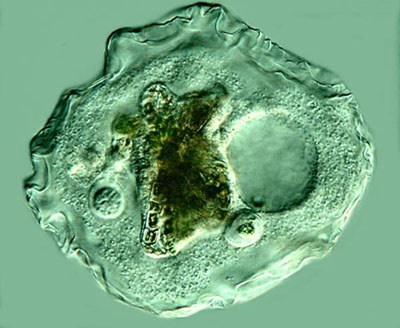
So, on to the amoebae. In the title, I referred to Thecamoeba as the Shar-Pei of protists. Theodor Jahn in his book How to Know the Protozoa refers to it as “the amoeba with a skin”. When it’s in a resting state, apparently leisurely digesting its repast, it takes on an almost knobby appearance. When it’s only moving slowly, it has a layered appearance.

However, when its actively seeking prey, it has ridges along the axis of movement, often four, but sometimes more. It is a voracious predator and not just on algae. I have observed specimens containing diatoms, bits of filamentous algae, and 2 or 3 Philodina rotifers. Here I added a drop or two of Neutral Red which, as you can see, vividly stains the filaments of the algae.
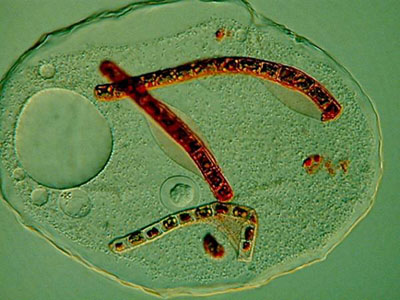
In the debris of the sample, I notice numerous empty parts of the cuticular envelope of rotifers, so apparently rotifers are a favorite entree on the menu for Thecamoeba. This is an image of another smaller amoeba in the same culture engulfing a bdelloid rotifer.
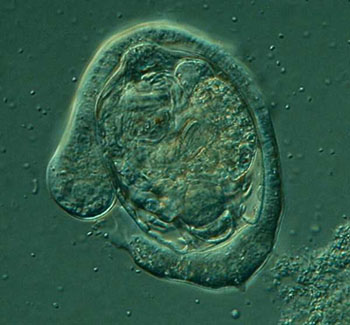
Another ambitious amoeboid feeder is a relatively wee critter, probably one of the smaller species of Mayorella, perhaps 30 to 50 microns in size, and either has “eyes bigger than its stomach” or a great deal of patience. I have seen specimens in the process of ingesting algal filaments 3 times its length producing quite a bizarre appearance.

Larger species of Mayorella are also abundantly present. This is a genus which could drive a meticulous taxonomist quite mad. In fact, there are a couple of species that could rather readily be confused with Thecamoeba. For Mayorella, F.C. Page, in his valuable little book, Freshwater and Soil Amoebae describes 22 species ranging in size from 12 microns to over 350 microns which provides room for a wide range of variation. Nonetheless, human consciousness is amazingly adept at pattern recognition and classification. The recognition of this remarkable capacity goes back at least as far as Plato, whose Theory of Ideal Forms was the first major attempt at a theory of classification. Once a child learns 10 or 20 kinds of trees, he or she develops a vague and elastic category about which things belong in the class of trees and which do not. With only a very little education, we become startlingly adept at classifying all kinds of things in terms of general categories. If our interest in a particular group of things becomes strongly focused, then we learn or invent all kinds of subcategories. However, it often remains extraordinarily difficult to specify the criteria of classification and virtually impossible to do so in an exhaustive and unambiguous way, even though one may be very adept at using such a framework.
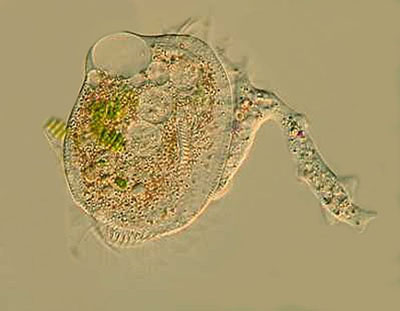
With organisms such as amoebae, it is indeed a matter of training the eye to notice details and patterns and to be able to organize them in such a way that one develops a kind of Gestalt impression that aids in classifying. The reason that this is important here is that for all the possible sorts of variations in the different species of Mayorella, one can develop a kind of “pattern sense”, so that you can say—ah, yes, a Mayorella, not a Thecamoeba, and certainly not Amoeba proteus nor Pelomyxa nor Dinamoeba. I can remember a time when I thought, if you’ve seen one amoeba, you’ve seem them all. This “pattern sense” is by no means infallible, but carefully nurtured, it is a valuable conceptual tool. Nature is, however, full of tricks and a single species can adopt a variety of forms either as a consequence of developmental stages as in the case of Pelomyxa or , in many species, as a consequence of adverse environmental shifts. When a culture gets old and stale, Amoeba proteus can demonstrate yet another facet of its protean nature. The study and classification of amoeboid forms can demand the microscopist’s full bag of tricks—careful examination of a significant number of specimens of each type, phase contrast, Nomarski DIC, fluorescence, dark-field, both simple and elaborate staining techniques, the use of the hanging drop method when feasible, and careful study of both photomicrographic and videomicrographic records. Sometimes even all of that is still insufficient and a species determination becomes possible only after an investigation of the organisms ultrastructure using the most sophisticated and expensive equipment and techniques of the professional researchers. However, that aside, there is still much which the amateur can enjoy and learn from investigating various species of amoebae. Thecamoeba is one example, but there are a variety of others which are equally interesting, but often neglected and many of them are fairy common in soil and moss cultures. Dinamoeba has pseudopodia tike tapering rods covered with minute thorns; Gephyramoeba has a voracious appetite that sometimes leads it to take on prey larger than itself as shown in the accompanying image. Here we see one attempt to ingest a Euplotes at least twice its size.
And to further confuse our urges to classify, there are at least two genera of amoeba with flagella! So, Power to the Proteans!
All comments to the author Richard Howey are welcomed.
Images by the author.
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK