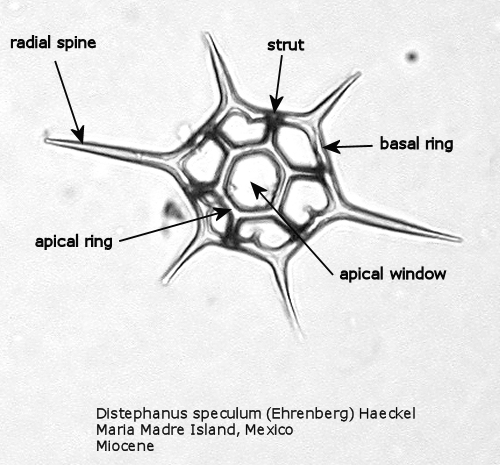
Figure 1. Maximum length = 65 microns.
|
Silicoflagellates Richard T. Carter, Hebei Normal University, Shijiazhuang, China |
Editor's note: The author has presented an update of this article in the February 2008 Micscape issue: 'Silicoflagellates 2: Addenda and corrigenda'.
Those of us who are “diatomaniacs” usually possess collections of recent and fossil marine strew slides. Some we make ourselves; others we purchase, perhaps from a current dealer like Klaus Kemp, or from antique collections that appear on such internet sites as eBay. Scanning these slides reveals not only the diatoms that are so dear to our hearts, but other siliceous microfossils as well, most notably radiolarians and silicoflagellates. It is my impression that we often recognize the latter when we see them, but pay them little attention. It is my intention in this little paper to suggest that this inattention is unwarranted: silicoflagellates are quite intriguing, the commoner ones are not difficult to identify, and there are good sources of information on the internet that can give us a reasonable understanding of these organisms.
The silicoflagellates are a group of planktonic protists, exclusively marine, that contain chromatophores for photosynthesis. In that respect they are like other “algae”. They possess a single flagellum, presumably to maintain proper orientation to the sun for maximum efficiency in photosynthetic production. Unlike other “algae”, however, they also possess pseudopodia – a fact that led several early scholars to classify them as “animals”. When we see a silicoflagellate on a strew slide, we are looking only at the skeleton of the organism, a supporting framework of hollow bars made of amorphous silica. Silicoflagellates are not unique, of course, in their ability to secrete amorphous, opaline silica: diatoms make an external “pillbox” frustule of such silica, while various scaled chrysophytes, most notably synuraceans, cover either the individual protistan cell or their colonies with ornamented siliceous scales (Cf. Wehr and Sheath 2003).
Silicoflagellates exist in the world oceans of today, but the number of extant species is problematic and controversial. The problem centers on our lack of understanding of what morphological variation in silicoflagellates actually represents. Many biologists feel that variation in extant populations mainly represents response to environmental variables, leaving its phylogenetic significance unclear. Accordingly, some workers believe that there are only three extant species; others believe there is only one! The fossil record is full of recognizably distinct forms, but we again have no idea what these variations mean in terms of phylogenetic taxonomy. Paleontologists have created many species, subspecies, and varieties, based largely on the criterion of stratigraphic value – and it is indeed the case that silicoflagellates are very useful in the correlation of marine sedimentary deposits. Such taxa are thus to be viewed as morphotaxa, fossil forms that are treated as different taxa as a matter of convenience. (For an excellent overview of silicoflagellate taxonomy, see Parkinson 2002.)

Figure
1. Maximum length = 65 microns.
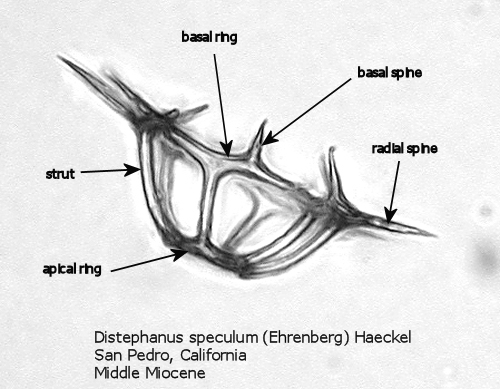
Figure
2. Maximum width = 63 microns.
Figures 1 and 2 are photographs of the silicoflagellate Distephanus speculum (Ehrenberg) Haeckel, seen from two points of view, with the main parts of the silica skeleton labeled. Although silicoflagellates first appeared in the Lower Cretaceous as highly varied (but relatively simple) skeletal structures (Cf. McCartney et al., 1990), by the Upper Cretaceous a “basic skeletal plan” had become stabilized. In the simplest forms, this consists of a basal ring, with or without radial spines and accessory basal spines. Later developments were the addition of struts supporting an apical structure of some sort, often an apical ring. Figure 1 shows a dorsal view of one of these more complex skeleta, and Figure 2 represents the lateral view. The basal ring is six-sided, with a radial spine at each corner. Two of these radial spines are longer than the other four, establishing a major axis. An apical ring is supported by six struts that arise from near the midpoints of the six sides, and the apical ring is also six-sided. This is a typical structural symmetry: the number of sides in the basal ring, number of radial spines (if any), number of basal accessory spines (if any), number of struts (if any), and number of sides in the apical ring (if any) are normally identical.
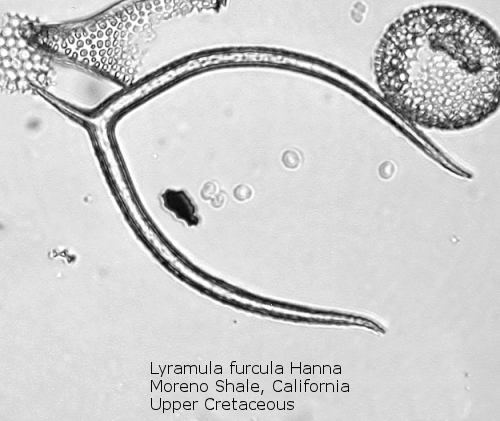
Figure
3. Maximum length = 94 microns.
An interesting exception to this basic skeletal plan is afforded by the genus Lyramula, seen in Figure 3. These primitive forms have no basal ring; rather, they exhibit two curving “arms”, basally joined, with or without a radial spine at the single corner so formed. This genus apparently disappeared during the great extinction event at the end of the Cretaceous. Forms described as Lyramula from the Cenozoic appear to be misidentifications of other structures, often basally-fused bristles of the diatom genus Chaetoceros.
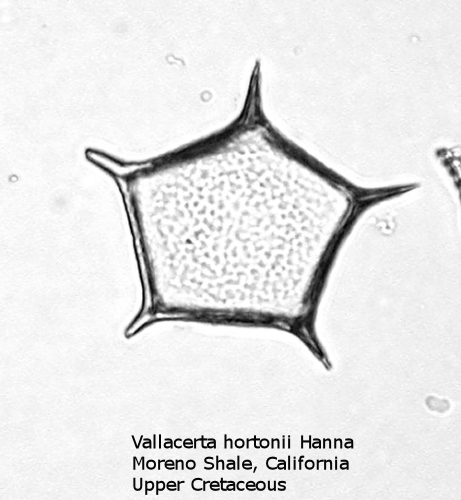
Figure
4. Maximum width = 52 microns.
A more typical Upper Cretaceous silicoflagellate is Vallacerta, seen in Figure 4. The basal ring has five sides, with a radial spine at each corner. The ring is filled by a thin, punctate plate of hyaline silica. The skeletal rods frequently break free, and it is thus not uncommon to find the thin pentagonal plates on a strew slide, looking like some unusual diatom.
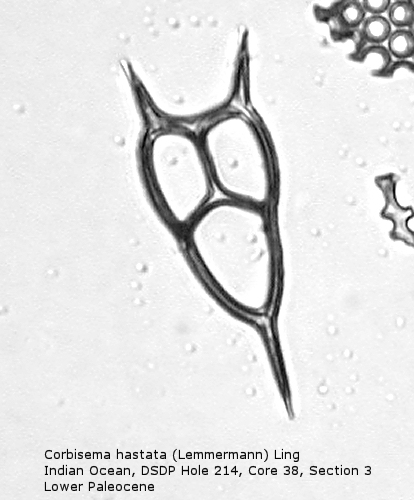
Figure
5. Maximum length = 49 microns.
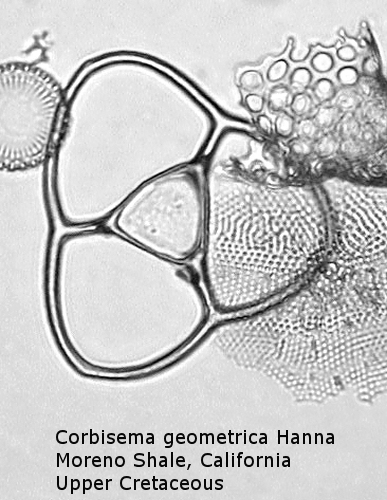
Figure
6. Maximum width = 56 microns.
Also appearing in the Upper Cretaceous is the genus Corbisema, generally comprising forms with a three-sided (often trilobate) basal ring and three struts. Figure 5 shows Corbisema hastata (Lemmermann) Ling, a very distinctive Paleocene silicoflagellate shaped like an isosceles triangle. Trilobate basal rings are produced when the three sides are constricted at the points of attachment of the three apical struts. Often the struts meet in a central point, but in the genotype, Corbisema geometrica Hanna (Figure 6), the struts may support a thin apical plate, rather like that of Vallacerta. A few members of this genus are bipolar, with or without two radial spines, and with a single arched strut that bridges the minor axis of the skeleton. (In this respect they strongly resemble the genus Naviculopsis; the latter, however, always has the basal ring laterally flattened where the bridging strut attaches.) Corbisema survived the Cretaceous extinction, and numerous forms have been described from the Cenozoic.
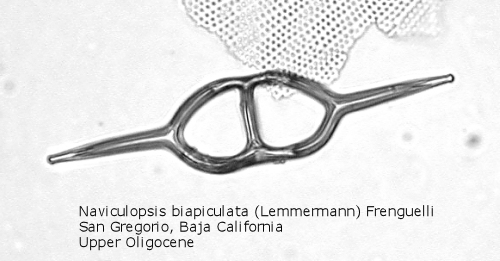
Figure
7. Maximum length = 85 microns.
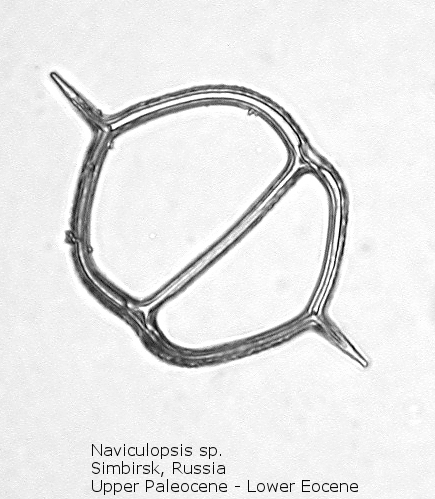
Figure
8. Maximum length = 76 microns.
A genus making its first appearance in the early Cenozoic is Naviculopsis (Figure 7). The basal ring, typically with two long radial spines, appears as an oval, with a single curved strut that bridges the minor axis of the skeleton. In many species the basal ring may be constricted at the points of attachment of the apical strut (Figure 8); as noted above, the basal ring is always laterally flattened at this point. At least one morphospecies has a long apical spine, rising dorsally from the center of the bridge, normal to the plane of the basal ring.
Figure
9. Maximum width = 65 microns.
Figure
10. Maximum length of side = 38 microns.
Figure
11. Maximum width of left specimen = 87 microns.
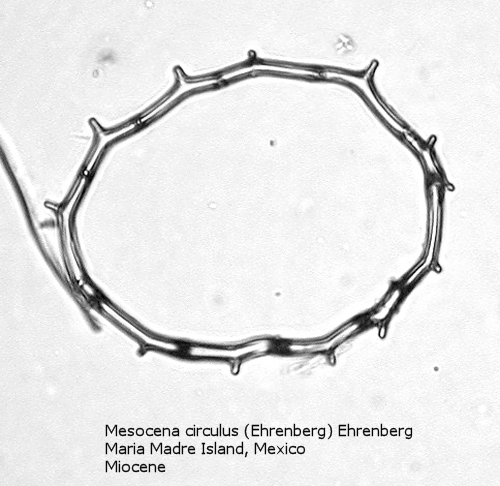
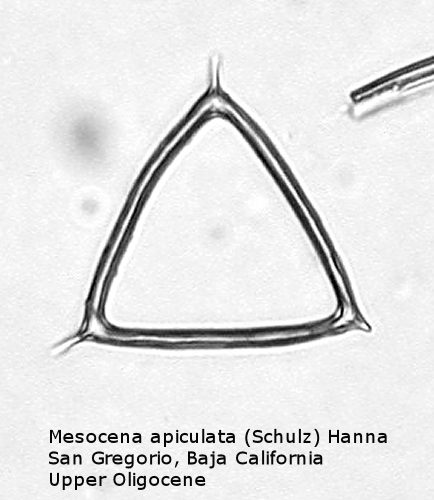
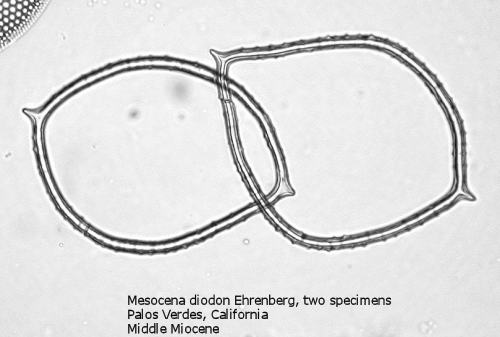
One of the most common genera in Tertiary deposits is Mesocena. This genus conforms to the basic skeletal plan of a basal ring, with or without radial spines, but with no apical apparatus. A common form in the Upper Miocene is Mesocena circulus (Ehrenberg) Ehrenberg (Figure 9). It shows a circular or ovoid basal ring without radial spines, but with numerous small apiculi. An earlier morphospecies, first appearing in the Eocene, is Mesocena apiculata (Schulz) Hanna, a three-sided species with three small radial spines (Figure 10). A larger species, also common in the Miocene, is Mesocena diodon Ehrenberg, with two radial spines and a strongly ornamented basal ring (Figure 11). (A junior synonym of Mesocena is Bachmannocena.)
Figure
12. Maximum length = 64 microns.
Figure
13. Maximum length = 61 microns.
Figure
14. Maximum length = 57 microns.
Figure
15. Maximum length = 64 microns.
Figure
16. Maximum length = 34 microns.
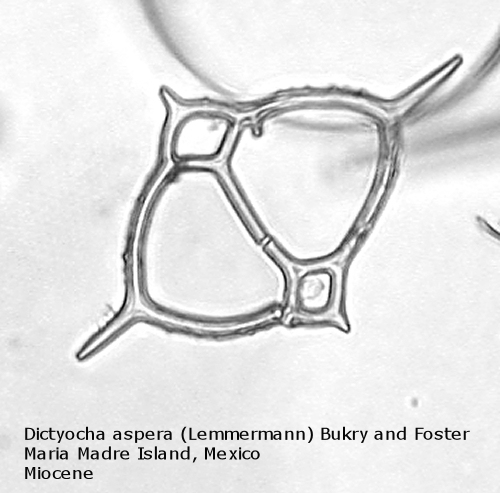
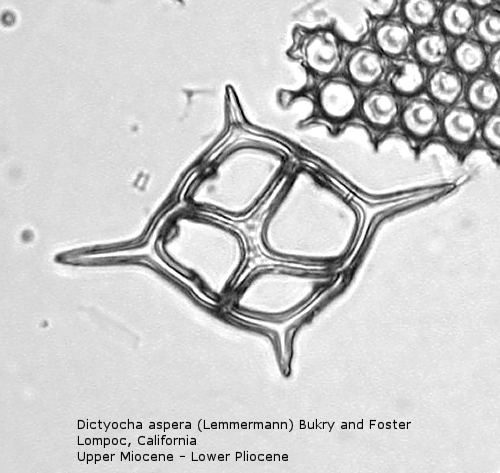
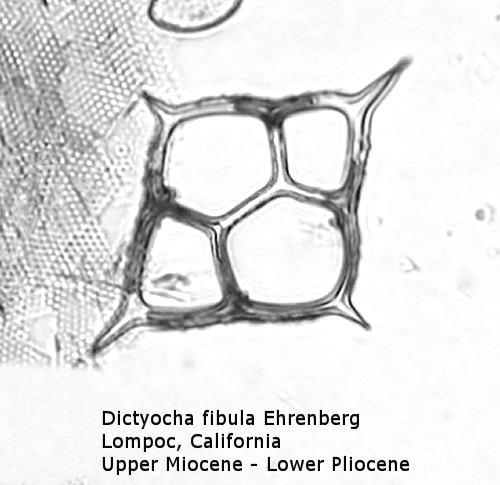
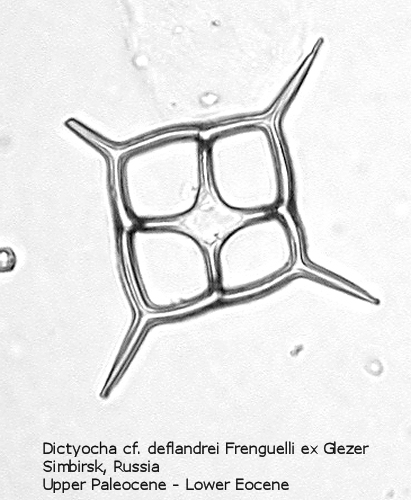
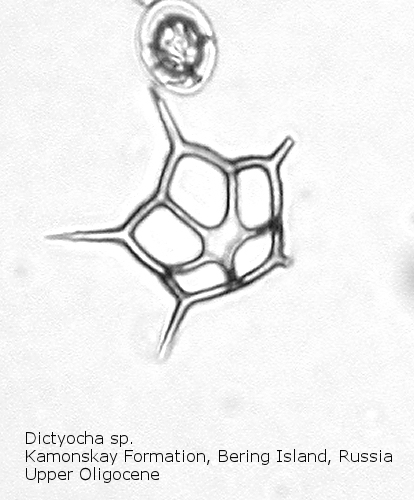
The first genus of silicoflagellates to be described was Dictyocha, erected by Ehrenberg in 1837. In modern practice this genus has been restricted by most workers to morphospecies that possess the basic skeletal plan, with lateral struts, but whose apical apparatus lacks an apical ring. In most Dictyocha the lateral struts support a simple apical bar, or bridge. Simple forms exhibit four-sided symmetry, but more complex forms may have an increased number of sides, often with a more complicated apical bridge structure. Morphospecies are distinguished on these criteria, as well as presence or absence of a major axis, and structural orientation of the apical bridge. Figure 12 is a photograph of Dictyocha aspera (Lemmermann) Bukry and Foster. This morphospecies has four struts arising from the basal ring, and the apical bridge is oriented parallel to the minor axis. The struts may arise at different positions on the sides of the basal ring; Figure 13 shows a specimen in which the struts arise near the midpoints of the four sides. This morphospecies differs from Dictyocha fibula Ehrenberg (Figure 14), in that the apical bridge of the latter is parallel to the major axis. Dictyocha deflandrei Frenguelli ex Glezer (Figure 15) is an early form with roughly equant radial spines and four struts that meet in a small apical cross, or plate. (Compare this illustration with that of Distephanus crux (Ehrenberg) Haeckel, below.) Figure 16 represents an Upper Oligocene form with five-fold symmetry.
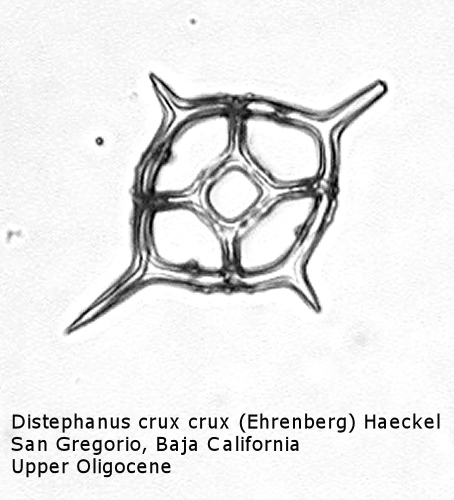
Figure
17. Maximum length = 51 microns.
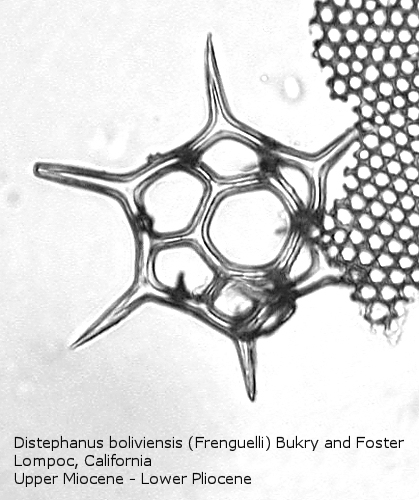
Figure
18. Maximum length = 63 microns.
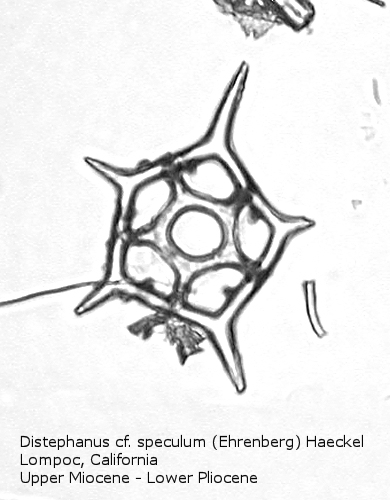
Figure
19. Maximum length = 50 microns.
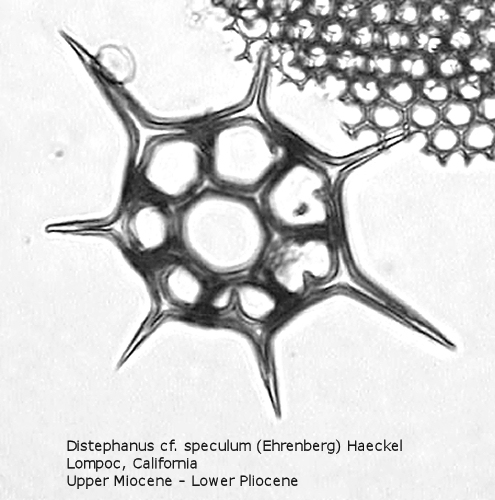
Figure
20. Maximum length = 66 microns.
Distephanus differs from Dictyocha in that the apical apparatus of the former is represented by a ring with a median window. The window ranges in size from a small, round hole, to a large, polygonal opening. A long-ranging morphospecies is Distephanus crux (Ehrenberg) Haeckel (Figure 17), a form with four-fold symmetry. Distephanus speculum (Ehrenberg) Haeckel, which we saw in Figures 1 and 2, represents a basic form with six-fold symmetry. This morphospecies has radial spines of unequal lengths, two longer ones defining the major axis and four shorter ones. Distephanus boliviensis (Frenguelli) Bukry and Foster (Figure 18) differs in that it is more robust, with equant radial spines. Distephanus speculum also has forms with five radial spines (Figure 19); note the unequal lengths of the spines. (Five-fold forms with equal radial spines have been described as Distephanus quinquangellus Bukry and Foster.) Similarly, there are forms with seven radial spines (Figure 20); again, note the unequal lengths of these spines. (Seven-fold forms with equal radial spines have been described as Distephanus septenarius (Ehrenberg) Frenguelli.)
There are numerous works on the internet, especially in publications of the DSDP/ODP, that can be readily used to identify silicoflagellates. Several of these sources that I have found most useful are listed in the References.
In conclusion, I think that I have learned a good bit about these interesting organisms in the process of writing this little article, and I hope that the reader will take a second look at the next silicoflagellate encountered on a diatom or radiolarian strew slide!
Comments to the author, Richard Carter , are welcomed.
References
Bukry, David, 1976, “Cenozoic Silicoflagellate and Coccolith Stratigraphy, South Atlantic Ocean, Deep Sea Drilling Project Leg 36,” Deep Sea Drilling Project Initial Reports, Vol. 35, pp. 885-917. (http://www.deepseadrilling.org/35/volume/dsdp35_appendixV.pdf)
Busen, Karen Eason and Sherwood W. Wise, Jr., 1977, “Silicoflagellate Stratigraphy, Deep Sea Drilling Project, Leg 36,” Deep Sea Drilling Project Initial Reports, Vol. 36, pp. 697-743. (http://www.deepseadrilling.org/36/volume/dsdp36_13.pdf)
Haq, Bilal U. and Anne Riley, 1976, “Antarctic Silicoflagellates from the Southeast Pacific, Deep Sea Drilling Project Leg 35,” Deep Sea Drilling Project Initial Reports, Vol. 35, pp. 673-691. (http://www.deepseadrilling.org/35/volume/dsdp35_37.pdf)
Martini, Erland, 1990, “Tertiary and Quaternary Silicoflagellates, Actiniscideans, and Ebrideans from the Eastern Pacific off Peru (Leg 112),” Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 112, pp. 157-173. (http://www-odp.tamu.edu/publications/112_SR/VOLUME/CHAPTERS/sr112_10.pdf)
McCartney, Kevin, Sherry Churchill, and Linda Woestendiek, 1995, “Silicoflagellates and Ebridians from Leg 138, Eastern Equatorial Pacific,” Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 138, pp. 129-162. (http://www-odp.tamu.edu/publications/138_SR/VOLUME/CHAPTERS/sr138_08.pdf)
McCartney, Kevin and Sherwood W. Wise, Jr., 1990, “Cenozoic Silicoflagellates and Ebridians from ODP Leg 113: Biostratigraphy and Notes on Morphologic Variability,” Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 113, pp. 729-760. (http://www-odp.tamu.edu/publications/113_SR/VOLUME/CHAPTERS/sr113_42.pdf)
McCartney, Kevin, Sherwood W. Wise, Jr., David M. Harwood, and Rainer Gersonde, 1990, “Enigmatic Lower Albian Silicoflagellates from ODP Site 693: Progenitors of the Order Silicoflagellata?” Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 113, pp. 427-442. (http://www-odp.tamu.edu/publications/113_SR/VOLUME/CHAPTERS/sr113_27.pdf)
Parkinson, Phil, 2002, “Ontogeny v. Phylogeny: The Strange Case of the Silicoflagellates,” Constancea 83, 2002. (http://ucjeps.berkeley.edu/constancea/83/parkinson/Dictyocha.html)
Wehr, John D. and Robert G. Sheath, 2003, Freshwater Algae of North America, Academic Press.
Related Micscape articles:
“Series on marine phytoplankton: The silicoflagellate Dictyocha” by René van Wezel, Micscape, February 2004.
Microscopy UK Front Page
Micscape Magazine
Article Library
Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of the Microscopy UK web site at Microscopy-UK.