 |
Thriving in the rain
extreme limnoterrestrial microhabitats
WALTER DIONI CANCÚN, MÉXICO |
INTRODUCTION
|
In 1952, almost exactly in the middle of the last century, Gene Kelly (use your browsers, for those who are very young) presented a magnificent film that remained in the first position of the musical comedies for many years: Singin' in the rain. I believe that if they had a voice, the organisms that I am going to describe would sing of happiness whenever it rained. But although they do not have it, I’m sure they take advantage of each drop of water that rains on Cancún, to awake, and to live swiftly for some hours. And due to the pluvial regime of Cancún, rain arrives every 6 months to them. During the time that the humid season lasts they thrive for short periods in the small pluvial water collections, scattered over the city, isolated from each other, sometimes only for 2 or 3 days, and return again to the dry condition, and so on, until the long dry season arrives, for them to sleep another 6 months. All the photomicrographs added to this work were captured in 2004 with the 0,3 Mpx DC3 Motic camera integrated in my National Optical microscope. Normal pictures were taken with my Canon Powershot A300 of 3,2 Mpx. All pictures were reduced to adapt them for the article. In many instances the background was replaced, to discard garbage and better display the subject Note: It would be a useful aid to read the previous articles a Key to the genera of Bdeloidea: Part 1, and 2
|
TEMPORARY HABITATS
|
How an experiment can be accidentally modified In a previous article I examined the microfauna and microflora of a rainwater reservoir in a tree of Cancún. . http://www.microscopy-uk.org.uk/mag/artoct04/wdtree.html The hole had only a diameter of 20 cm x 6-8 cm of depth, but it supported, in spite of having been filled only a few days before, a modest but interesting list of species, some with a very high density of individuals.
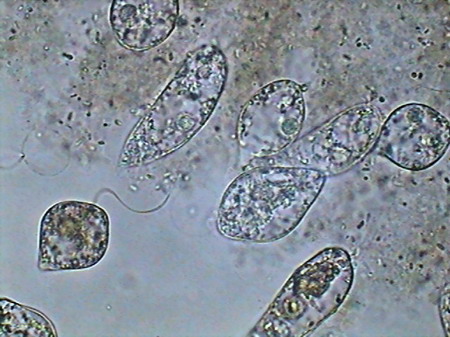
Here once lived Astasia, Khawkinia, Euglena, Dileptus, Halteria, Vorticella, and there were also vestiges of previous occupants like Centropyxis and a Bdelloidea, prob. Habrotrocha) see the previous article.
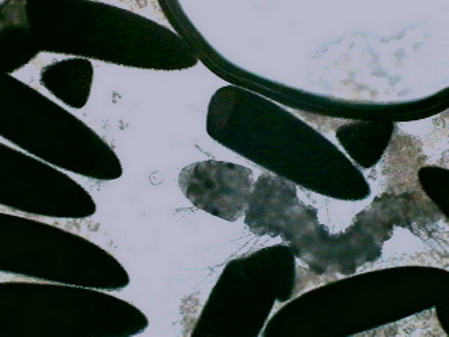
Astasia sp. mounted in GALA, fresh and not stained. After ten days, without disappearing, the microfauna had a much lower density, perhaps by competition for the limited existing resources with a great population of mosquito larvae recently hatched, or because it was consumed by the same larvae. Fifteen days later the hollow was completely dry. The cycle, from filling to dry, was of 30 to 35 days. From the hundreds of hatched mosquito larvae, probably only a few tens arrived at the adult stage.
recently hatched mosquito larvae
These biocenosis are being studied with interest, as much in nature as in the laboratory, because on one hand they are true models on scale of more complex, extensive and lasting biocenosis, and secondly because they really are one of the most difficult to eradicate sources of annoying and dangerous mosquitoes. In the neighborhood of Cancún they exist, although they are hardly seen in the city, the mosquitoes that transmit malaria, and dengue. One of the possible experimental approaches is not to depend on rains to maintain with water the natural holes, and another one is to even generate manageable artificial “holes” in the laboratory. For both tasks it is necessary to have rainwater at hand. Rain in Cancún is seasonal, with a very extensive dry season, and a not so long wet season, with heavy rains; so, to have a permanent source of rainwater it is necessary to gather it with sufficient anticipation. With the intention to make some short experiments (at the amateur level, it is clear) on these micro natural and artificial biocenosis, I gathered about 60 ltrs of rainwater, collected on a canvas suspended with that intention. The canvas formed a sink, which was washed, discarding the water collected the first two days, and from which water was later drained directly to three carboys 20 ltrs each. Carboys were of plastic with a very clear blue color, and their original destiny was to contain purified commercial drinking water.
The mystery microbiocenosis Four months later, already in the dry season, while I siphoned water to fill some experimental containers, I observed the presence of small pieces of a green-blackish 'felt' whose origin was, as it could be verified, a thin and extended bioderme developed over the container bottom. Thinking it could be cyanobacteria I examined some samples with the microscope. In fact what I found was a quite complex biocenosis. I verified that it was equal in the three carboys, which allowed me to suppose that it had been originated in cysts of organisms contributed by the wind to the rainwater gathered on the canvas.
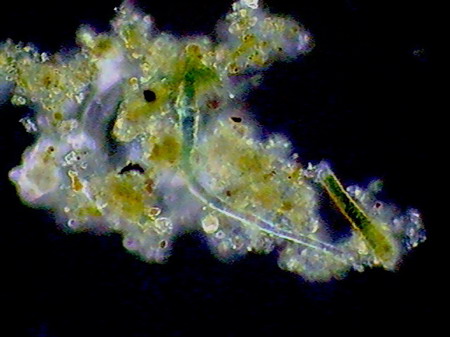
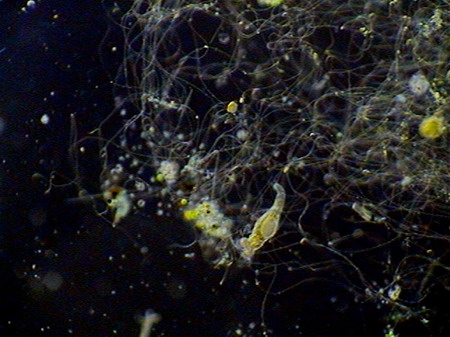
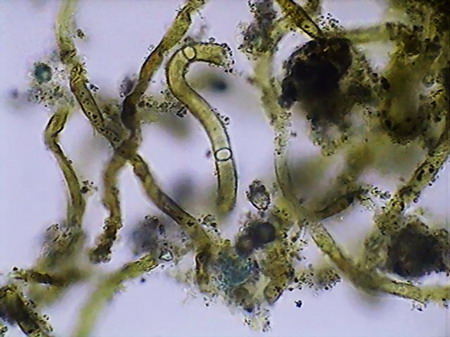
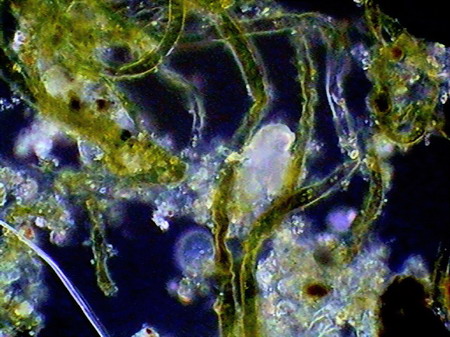
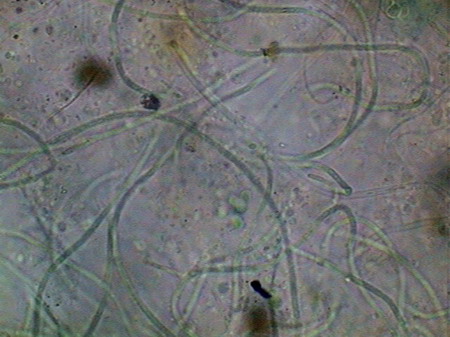
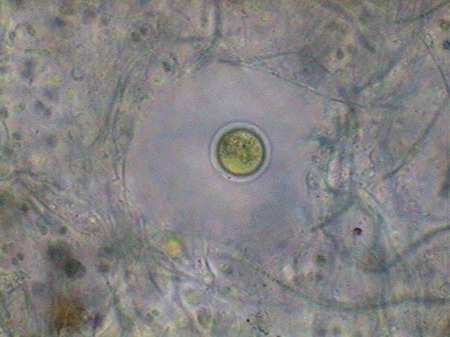
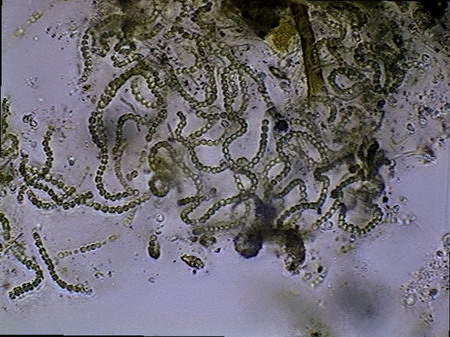
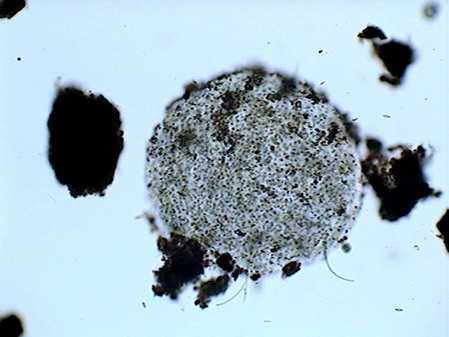
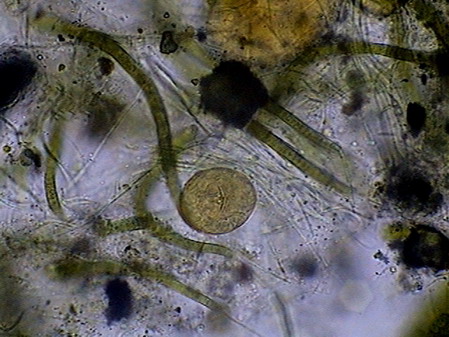
Beggiatoa, a filamentous bacteria conglomerate eubacteria, cyanobacteria and microalgae
rotifers exploring the substrate
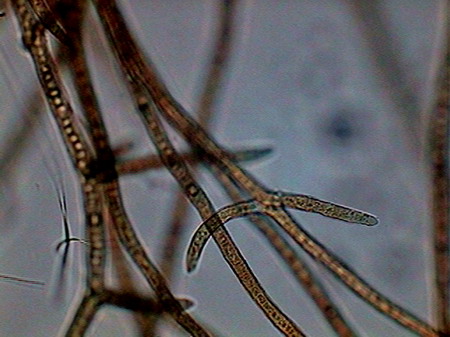
Vegetal felt was a thin, but dense and intricate growth of filamentous bacteria and cyanobacteria. Evidently they also existed, although no visible directly with the low power, multitude of small bacteria, denounced by the mucilaginous atmosphere they produced (see pictures above).
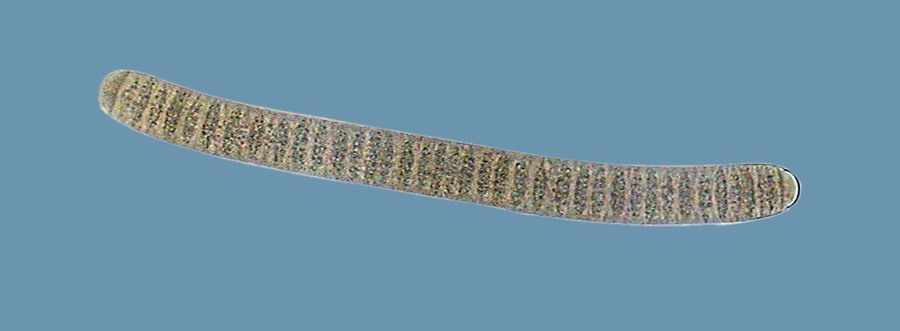
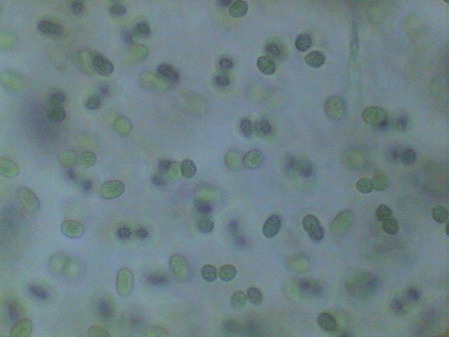
I believe that observed Cyanobacteria belong to at least 14 different genera (Anabaena, Beggiatoa, Calothryx, Scytonema, Microcystis, Gloeocapsa, Chroococus, Aphanotece, Nostoc, Leptolyngbya, Lyngbya, Oscillatoria, Chlorogloeopsis, Rivularia and others for which it was impossible for me to locate a probable identification…. For some genera there were present more than one species). It must be understood that I am not a botanist and not, and less, a bacteriologist or a phycologist, and that my only comparatives images and information source, on this theme, is the INTERNET, and the reason why, although I have made my best efforts to determine the genera, except in cases so typical as Nostoc, or Lyngbya , by ex., I must thank very much any corrections that a specialist who reads this work could contribute to improve this modest gallery. Those specialists who I tried to consult, did not respond to my request. So, these identifications are all only tentative.
Oscillatoria
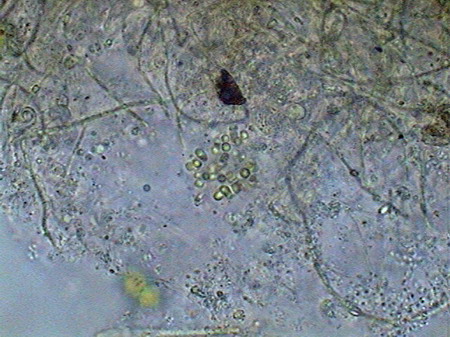
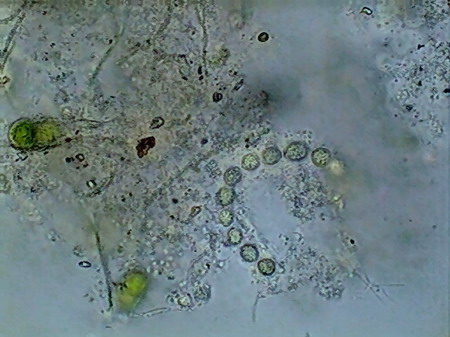
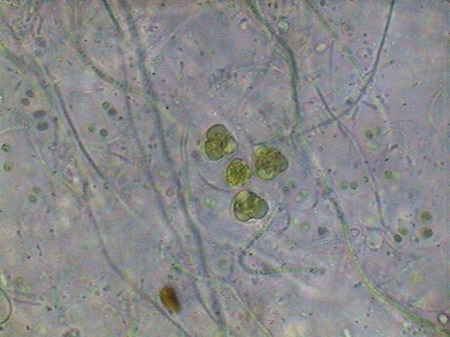
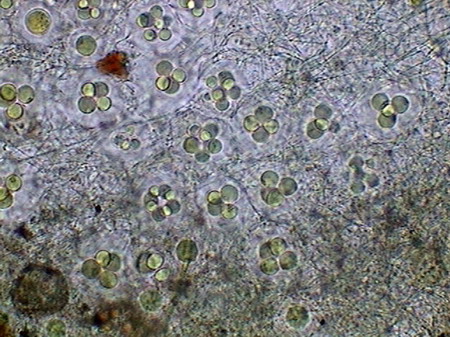
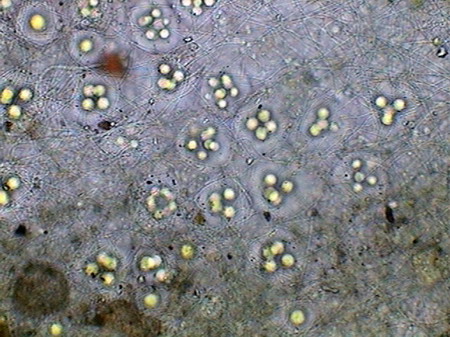
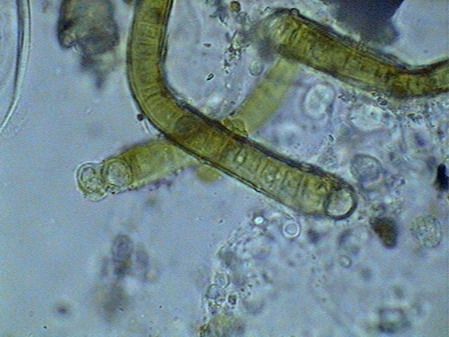
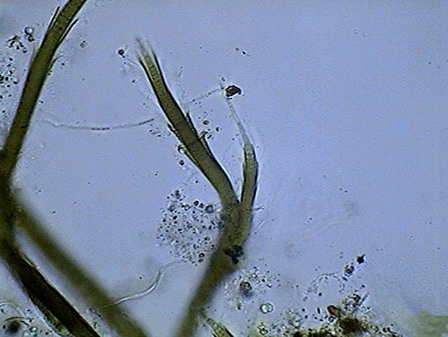
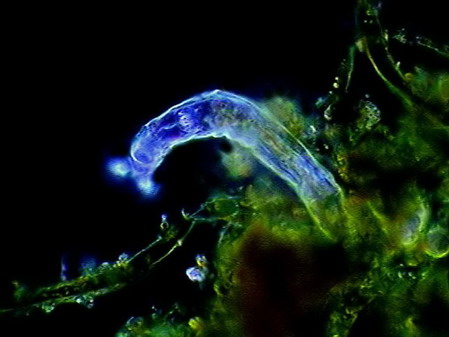
The microfauna Consuming mainly the bacteria and the detritus that cover or surround this material, were 4 species of rotifers (a species of Rotaria, another one of Philodina, an Adineta, and a Phylodinavidae). Cysts of them, as the ones shown in the two following pictures surely are dragged by the wind, when the habitat is dry.
When the habitat is wet the species are active and reproducing, as shown by the eggs in its interior, or the ones deposited between the vegetation.
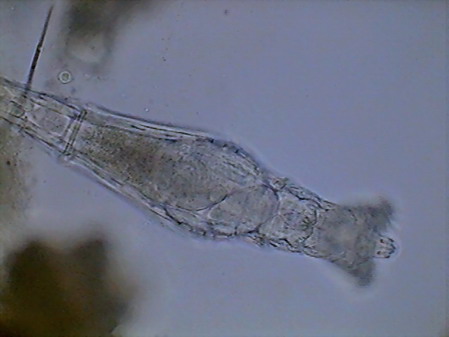
Rotaria, 2 rostral eyes, 1 young embryo Philodina, 2 brain eyes Samples of this biocenosis, transferred to the laboratory in covered containers, to avoid contamination and mosquitoes, stayed stable for more than a year, with later aggregates of some contaminating agents (a Cephalodella, and later some small ciliates)
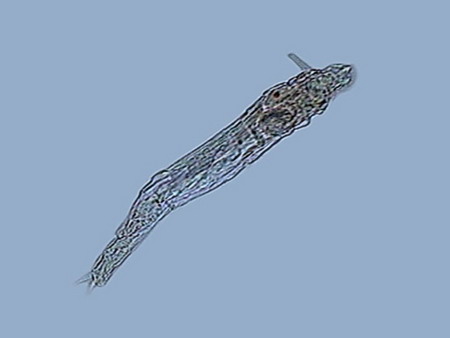
At least the species of Phylodinavidae seems to be new. The most similar genus seems Abrochta. If it would be, it would be easily distinguished from the other two now recognized in this genus. It seems also that the Adineta species is a new one. ROTIFER’S BEHAVIOR Genera can be easily identified even at low power. Philodina swims rarely. It stays clinging permanently to substrate while it extends all its length, and unfolds its absolutely typical trochae. Most of the time, it is filtering bacteria suspended in the water, as their trochal activity, their oscillating movements, and their frequent contractions demonstrate. At 100 powers their eyes can be easily distinguished, over the brain. Ovovitelogens are clearly visible, and often an egg almost mature, with a big nucleus. Rotaria can fasten itself to vegetation and act more or less like Philodina; but even fixed and extended, its characteristic proboscis identifies it. Very frequently it loosens its attachment, and uses its trochae to swim and to collect food, as the tireless activity of its mastax demonstrates. During swimming it bears the rostrum extended with its characteristic eyes at the end (visible with 100x, but most clearly with 400x) and the lamelas unfolded. It is also common that it closes the corona, and crawl on the substrate, giving to its head a long tapered profile where the retracted ciliated discs are clearly distinguished at the base of the always extended rostrum. Their ovovitelogenes are small. Normally it has in its interior a small, rather cylindrical egg, with blunt ends, and a bigger developed and movable embryo. Adineta has a size smaller than the two previous species, and a special shape and mobility. It remains generally extended, running on the substrate at great speed, propelled by its cephalic ventrally ciliated field, sweeping food from it at the same time. The flat tape-like head and trunk, the narrow neck, and the always extended foot of medium length with two small spurs laterally open, makes it unmistakable. When eating, it flattens on the substrate, showing its flexible and depressed profile, with tapered shape, not a cylindrical one like the two previous species. The Phyllodinavidae (whose taxonomic position is still uncertain) is a species whose atypical behavior immediately distinguishes it. It is small but robust, and normally it crawls, permanent and untiringly, between the handfuls of cyanobacteria of the substrate, which makes photography almost impossible. In order to move, it extends and retracts its relatively short foot, which generally forms a strong angle with the trunk. This is quite rigid, and inclines like a unit due to the existence of rectangular cuticular plates. Uniting this to the gross head, crowned by the big rostrum, with strong cilia at its end, also inclined to explore and to scrape the substrate with an alternative movement of capture and traction, it is difficult not to find that it has a comical aspect. Aside from the zoological distance, the movement closely remembers a chicken pecking for its food. NATURAL PLUVIAL BIOCENOSIS It is commonplace in Cancún that after rains, there form little pools in the ground, the sidewalks, and even in the street, that could maintain water for a couple of days, and that often will be filled again with the following rain, before they are dried up. Also it is common that at their bottom a mud layer forms, in whose surface cyanobacteria develops, easily detectable by its black-greenish color. There are also cyanobacteria in places that receive permanent (or at least very frequent) dripping, from some water pump, or from a failing faucet, installed to feed hoses for garden irrigation, etc.
Various photos of extreme microhabitats The previous experience took me to investigate these sites, and I verified in all of them biocenosis very similar to the one developed in my carboys. I even identified on the roof of a nearby house the one that, by its specific composition, surely was the source of the cysts that were seeded by the rain in my carboys. During the rains a layer of water from 2 to 3 cm collects in this ceiling, and evaporates in a couple of days. The white reflecting bottom, and the heavy direct sunlight must raise the water temperature, around 2pm (14: 00) to a very high level.
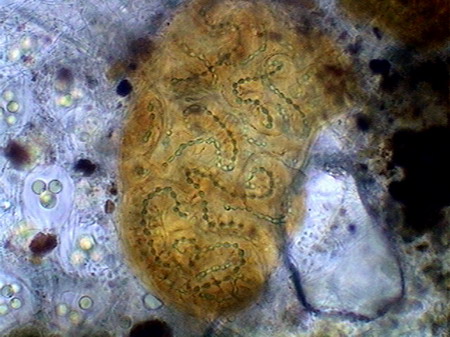
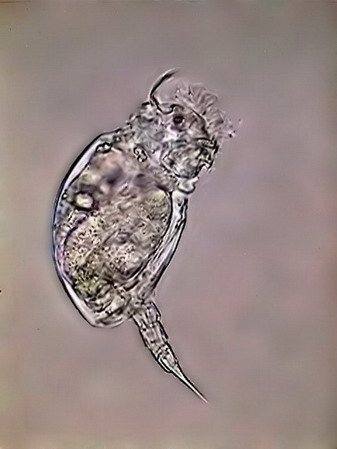
The list of rotifers species although always included some of those already found, reached a greater diversity, incorporating two Habrotrochidae (mainly Habrotrocha and cf. Otostephanus), a Mniobia, one second Philodinavidae (Abrochta) and another pair of bdelloids difficult so far to identify, although they probably are also Habrotrochidae. The Habrotrochidae family, characterizes itself by an apparently sincitial stomach, without the ciliated lumen which the Philodinida or the Philodinavida have, and in which the food is included in spherical vacuoles that give to the stomach the aspect of a bag filled with small greenish balls. Habrotrocha moves like a Philodinida, but it has a very short foot, a wide and oblong belly, and a longer and thinne neck (as also is the buccal tube), with a corona of hardly a little greater diameter. These characteristics make it almost immediately differentiable from the Philodinidae. The species found so far produces no capsule or housing, as described in other species. Their behavior is similar to that of Philodina: it remains stretched and feeding most of the time, but seldom swims. I could not take an acceptable picture of this species Otostephanus (or what is a very close genus) differs because its corona seems to have one double troca, and by a longer and differentiable neck. It has a morphology and behavior very similar to that of Habrotrocha. But is more sedentary. Mniobia surprised me at first by its general aspect during swimming, with its two dorsal eyes behind the antenna, which made it seem a Philodina. But a few moments of observation differentiate it quickly. The first segments of their torso are distinctly separated from the neck, are more rigid and the whole cuticle of the torso is more rigid. In addition, unmistakably, does not have toes in the foot but an adhesive disc. Abrochta is a small, agile species, with the typical genus organization, but this population has individuals much more thin those Abrochta intermedia , the species whereupon it must be compared, and to which it probably belongs.
cf. Otostephanus Mniobia
The unidentified specimens (at least two other species) are also Habrotrochidae, but I have not been able to define the number of fingers, nor the position and forms of spurs in the foot. They are shy species, generally hidden inside the vegetal mass. I could not obtain good comparative images. In all these natural biocenosis they are also some monogononta rotifers, (Cephalodella, Colurella) in much smaller quantity, and several small ciliates species.
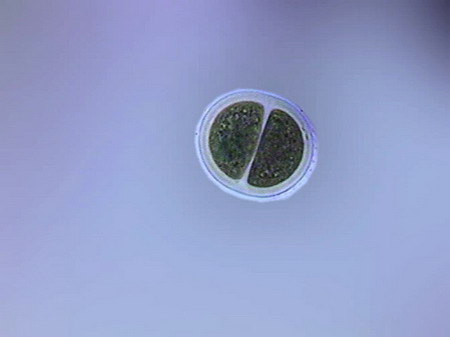
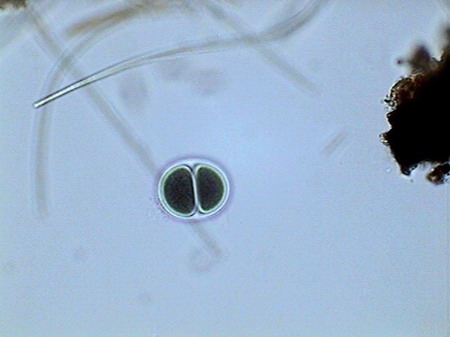
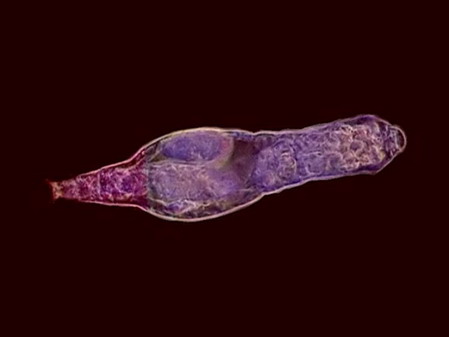
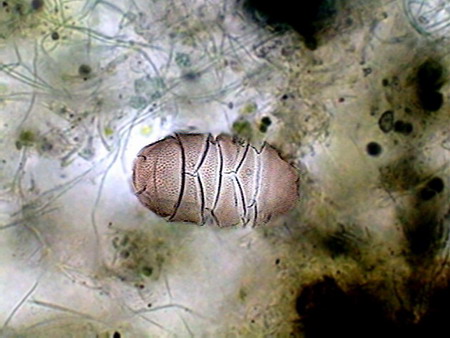
Colurella In one of the samples I found vestiges of a quite dense population of a tardigrade (Echiniscus). But the population was dead, leaving only his empty cuirasses, as the one shown below, before I could see them alive.
Until now I have altogether identified 8 (perhaps 9) genus of Bdelloidea adapted to survive in an extremely hostile medium, plus a tardigrade and a couple of Monogononta genus. Due to the location of all these biocenosis and to the substrate in which they are based, it is evident that, although I could not measure pH, this must be very high and the water must have a high calcium content. The action of the wind and the intense sunlight, dry in a few hours most of the investigated habitats. All this microfauna, and the microflora that provides them with food and shelter has a few hours to develop and complete their vital cycles. Their bodies support enormous biochemical tensions. Then it is not strange that species like all those here mentioned can be cultivated for very long periods, even in the relatively crude conditions of my laboratory. All of them are candidates to become laboratory models for the biology of their genus. NOTE: All cultures, which were established for more than a year, and maintained the identity and abundance of species of the original biocenosis, were lost during Hurricane Wilma in 2005. I hope to recover some populations in the rainy season that just started this year, to publish the new species, and to distribute some samples to laboratories qualified to fully utilize the experimental potential of these populations.
Comments to the author,
Walter
Dioni
, are welcomed.
© Microscopy UK or their contributors.Published in the July 2009 edition of Micscape. Please report any Web problems or offer general comments to the Micscape Editor.
Micscape is the on-line monthly magazine of
the Microscopy UK web © Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk with full mirror at www.microscopy-uk.net .
|