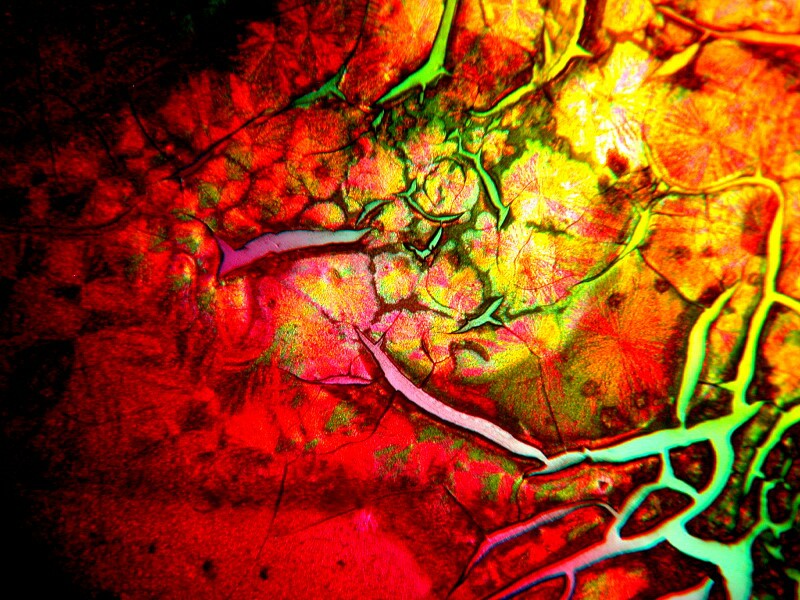
|
A Lazy Summer Gallery of Crystals by Richard L. Howey, Wyoming, USA |
I HATE heat above 72 degrees Fahrenheit! O.K., I can just tolerate 75 but, thatís why Iím an atheist. I couldnít abide the eternal heat of Hell and I quite understand that thatís the whole point of absolute punishment, but I regard that as a totally insane, twisted, perverted eschatology! So there!
When we first moved to the high plains of Laramie (7,200 feet) 47 years ago, a one day peak temperature of 80 degrees F. was mind-boggling and for many years thereafter, a temperature of 90 F. was simply unthinkable. Well, the forecast for tomorrow is for a temperature of 91 F. I describe this as a climatological obscenity.
I mention this by way of explanation. I had the text of a perfectly nice article on hairs and fibers finished and just needed to take some photographs to complete it for this issue. However, of the last month, it has been in the mid-80s to 90 F. up in my lab, so no pictures and I couldnít finish the article. My brain melted. However back in April and May, when the temperatures were decent, I took a lot of photographs of crystals, some of which you saw in my April Micscape article ďAnother Gallery of Polarized Crystals.Ē So, steel yourself, because thatís what youíre going to get this month too. I finally got desperate enough to contact a contractor to install some air-conditioning in the upstairs so, I am hoping that in a week or so, I can begin to retrieve the vestiges of my sanity and start doing some serious research. In the meantime, youíre just going to have to put up with some more crystal images and few very brief comments.
The first image is a mixture of Sodium Bicarbonate (Baking Soda) and Sodium Silicate (Water Glass). Water Glass used to be used as an egg preservative by coating the egg with this chemical ďmembraneĒ. It has peculiar properties and almost always produces odd wrinkles when mixed with other chemicals. You can see some here. Generally itís advisable to dilute it, otherwise you get chunky bits that tend to fall off the slide.

The next two images are taken at exactly the same point on a slide which contains a mixture of Nickel Sulfate and Urea. A bit later, Iíll show you some more examples of the very nice results which this combination can produce. The shift of colors is a consequence of a very small shift in the position of a rotary quartz compensator.

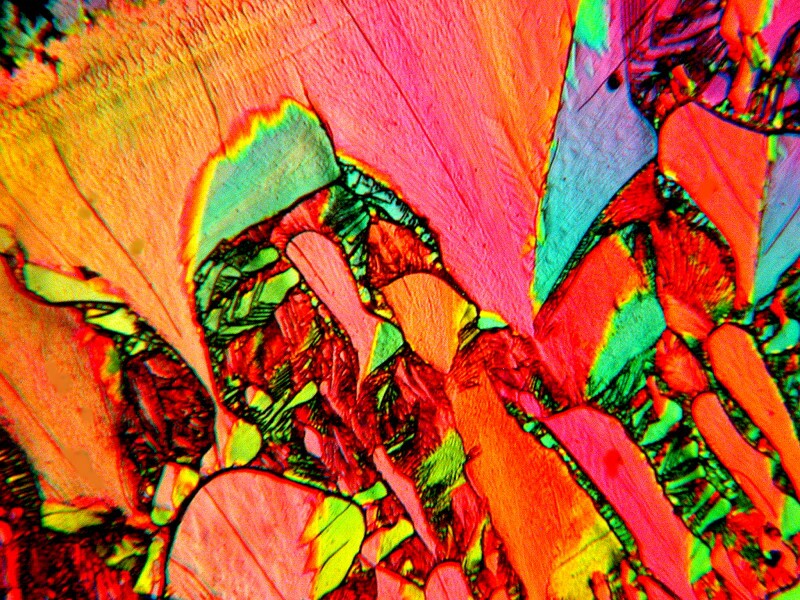
Next we encounter a modernistic piece of stained glass from a mixture of Nickel Sulfate and the biological stain Neutral Red.
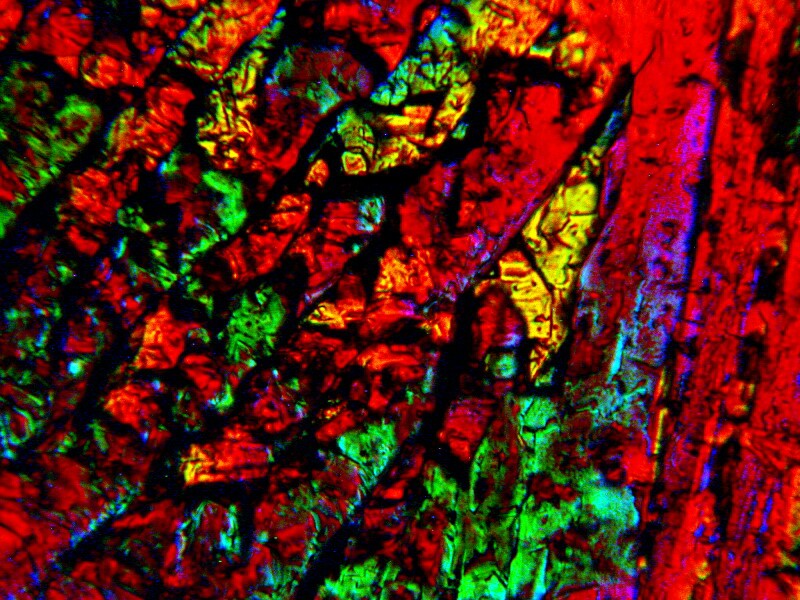
From time to time, I experiment with adding bits of calcareous material from an invertebrate to Ascorbic Acid which will dissolve it and then often produce very striking crystallization. The one below is the result of adding a bit of a ground up sea urchin spine. Iíll show you some other examples a bit further on.
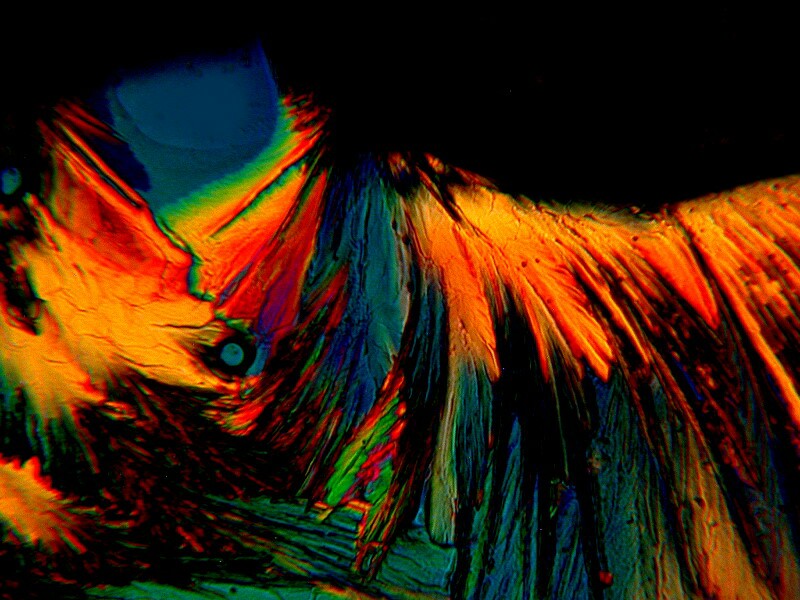
Sometimes ordinary household items produce some surprising results. In this instance, I mixed a bit of Borax with Ascorbic Acid which produced an interesting contrast of crystal types.

On occasion, more elaborate combinations will produce a startling variety of forms on the same slide. The two images below, which are a mixture of Anbesol (a toothache medication), Boric Acid, and Stevia (a natural sweetener), illustrate this nicely.
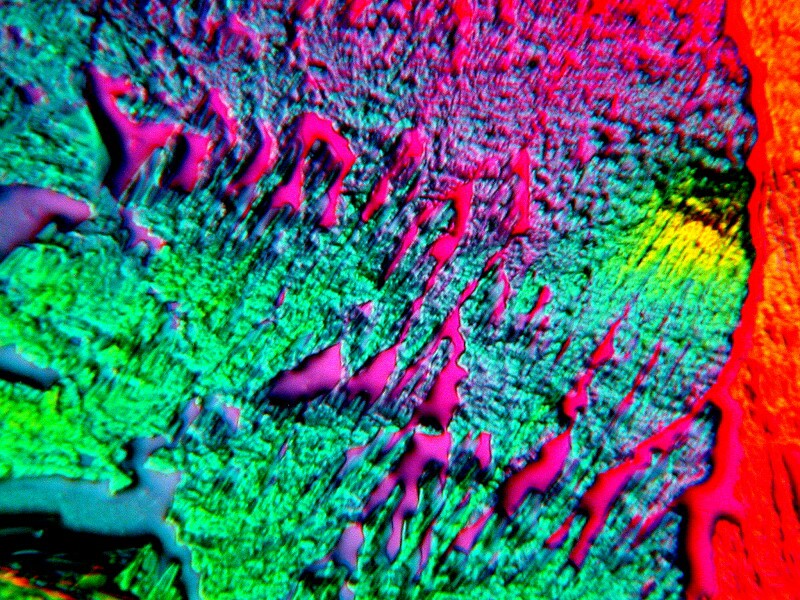

Some other items, which you might find in your medicine chest, can provide some unexpected and quite dazzling results. Consider this mixture of Ibuprofen, Ascorbic Acid and a drop of Mercurochrome.
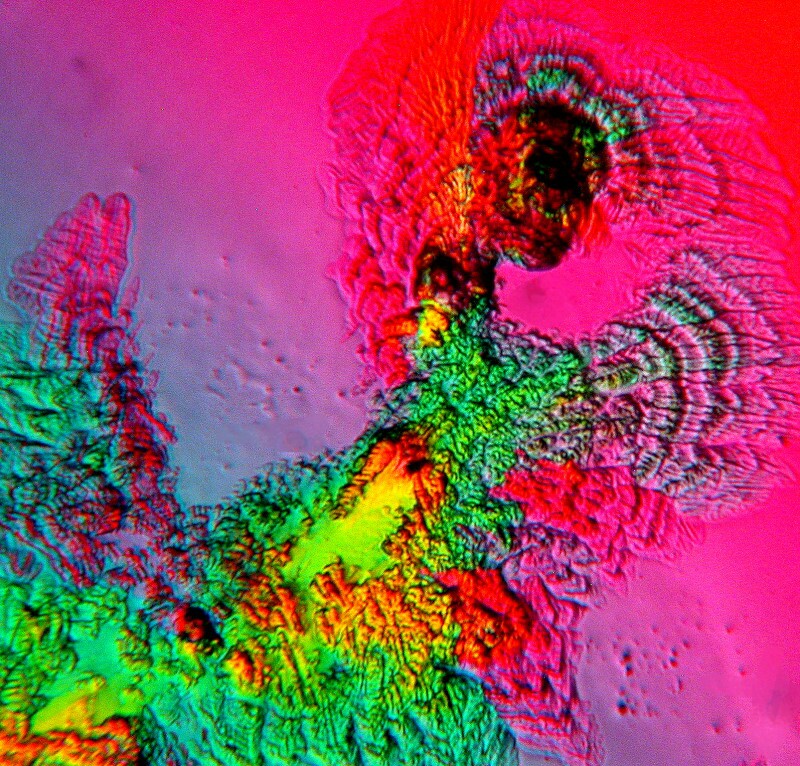
Or this one of Imodium and Ascorbic Acid.

Occasionally, one finds a mixture of chemicals that consistently provides interesting, but varied results and these can be a delight to explore. I mentioned earlier that Nickel Sulfate and Urea is such a combination. So here are three examples from a slide where the view is the same in each case but, there has been slight rotation of the quartz compensator.
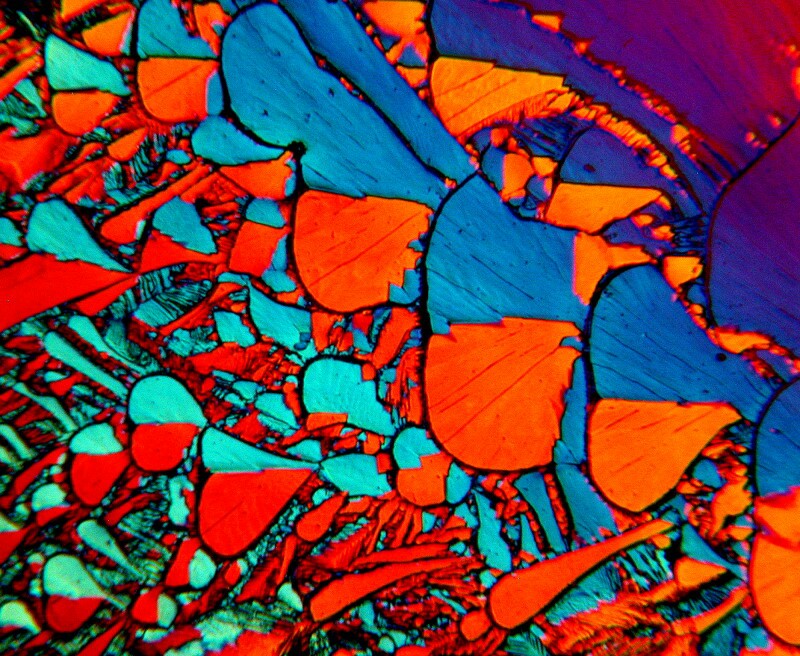
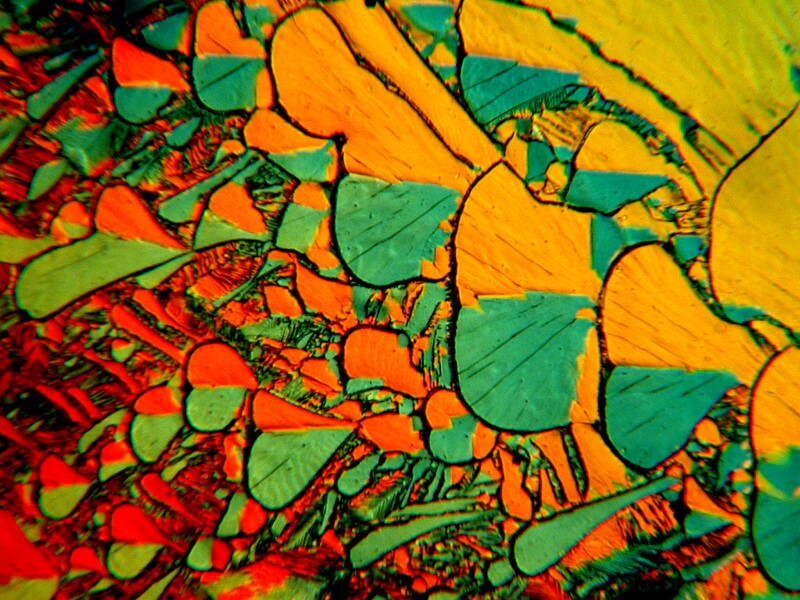

I find this pattern very pleasing and also take delight in the color shifts all of which are attained by the manipulation of light.
I mentioned before that on the same slide, one may find variations of such magnitude that it is difficult to believe that the same combination is producing such different results. Here is a case where, in the first image, this mixture of Anbesol and Sodium Silicate displays an alien crystalline bird for us and, in the second image, it looks almost like a bouquet of crystals lying on colorful carpet composed of a rich, luxurious fabric.

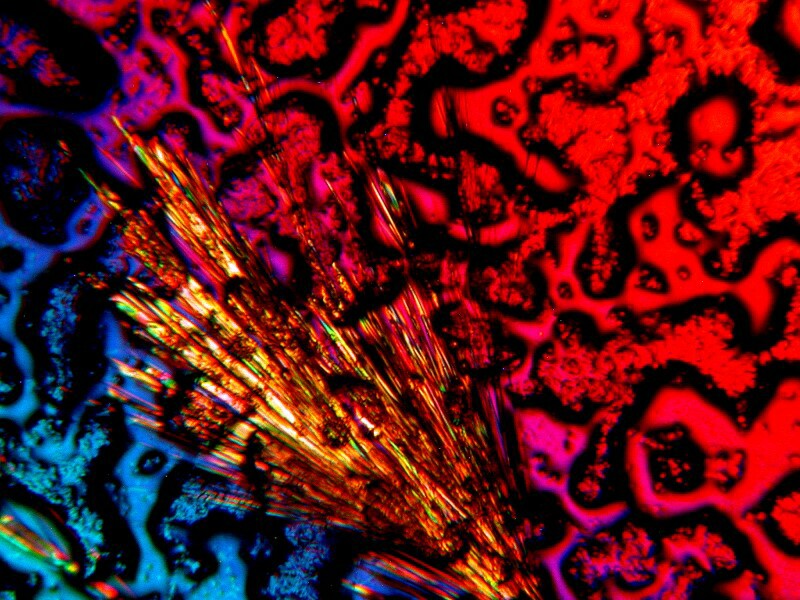
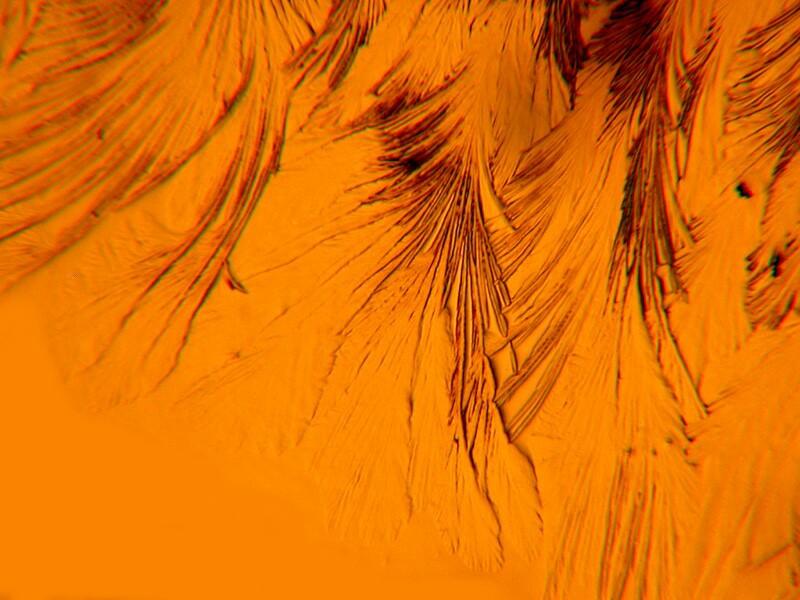
Now, letís return to something which I also mentioned previously, namely the use of invertebrate calcareous material with various chemicals. Again, I am using some crushed material from the spine of a sea urchin. The first image is not polarized and looks rather drab. The second is polarized and the quartz compensator is also in the light path producing a splendid, feathery display.

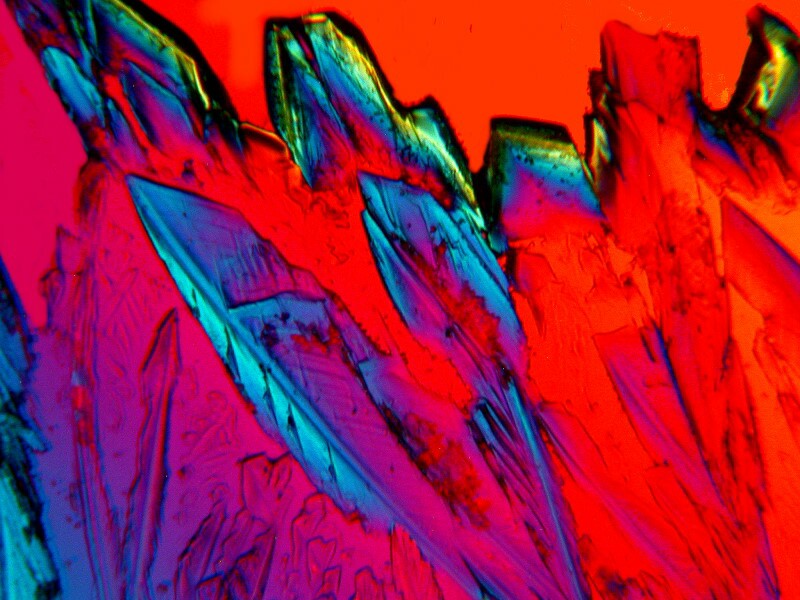
The next two utilize the urchin material, but the first one is mixed with Copper Sulfate and the second with Ferrous Ammonium Sulfate.
The copper produces strong, angular crystals, whereas the iron present us with a massive, ridged, layered block.
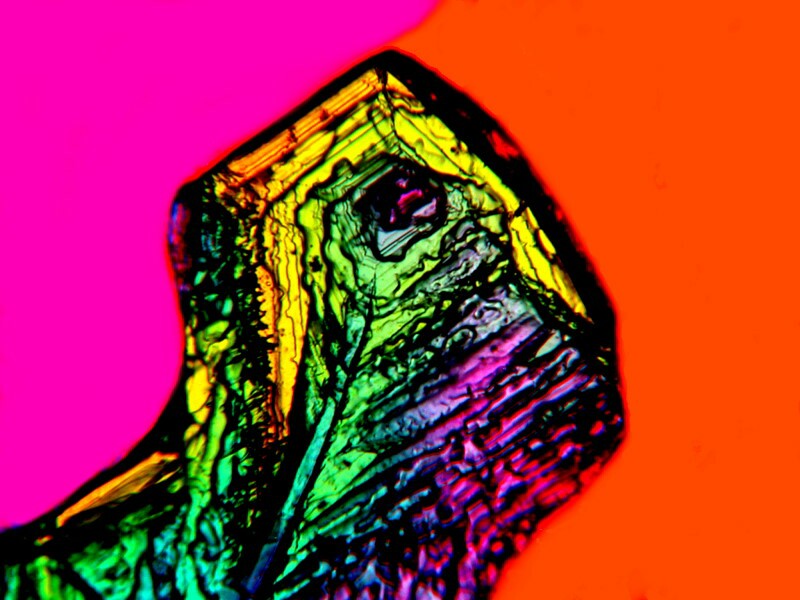
It is enormously satisfying when you are experimenting with combinations and come across one that produces totally unexpected results. This mixture of Copper Sulfate and Stevia I find quite wonderful in that the images look like some bizarre sort of alien calligraphy. Iíll show you 3 examples.


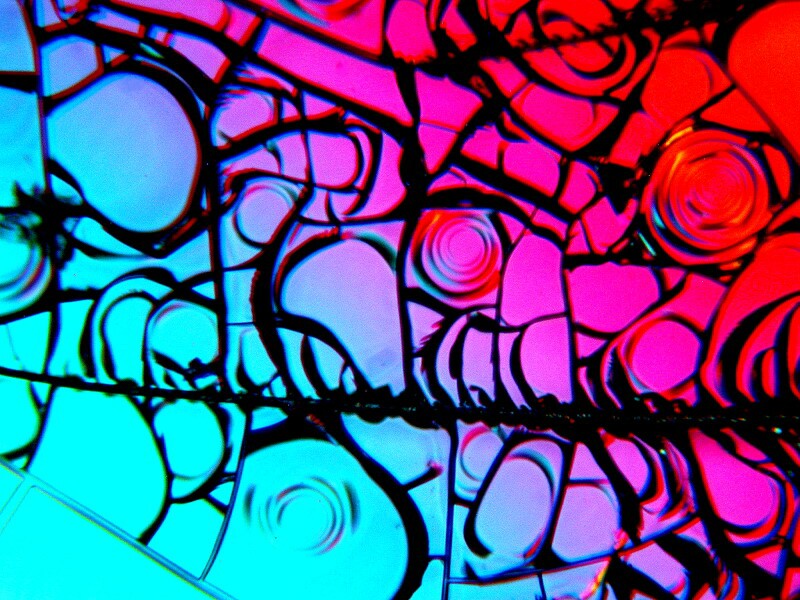
Finally, some mixtures produce images that seem both geometric and structural in form. This is a combination of Urea, the biological stain Orange G, and Stevia.
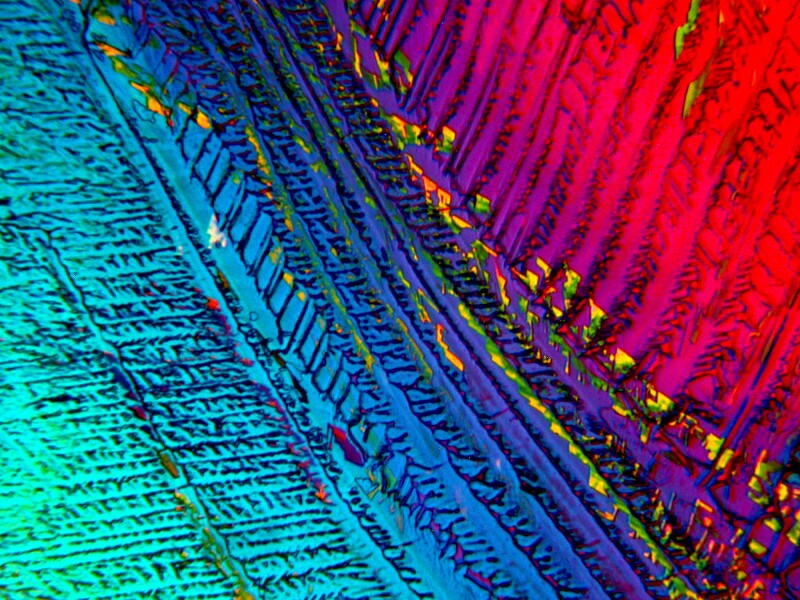
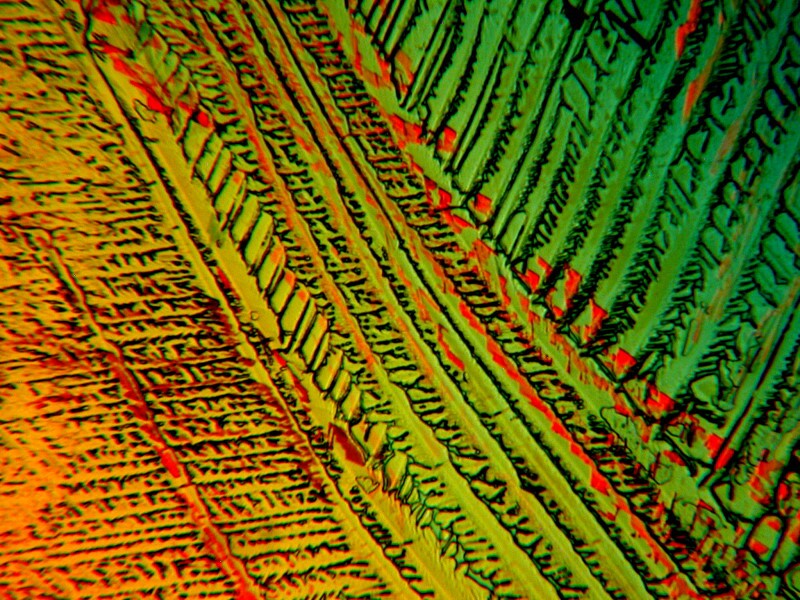
I hope you enjoyed this brief excursion and that you will be kind and pray for snow.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Published in the July 2013 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk .