
|
A Tribute to Brian Johnston by Richard L. Howey, Wyoming, USA |
I recently learned that Brian Johnston has decided to retire as a contributor to Micscape to pursue other interests. I know of him almost exclusively from his publications on Micscape and a few e-mails which we exchanged over the years. He was a chemistry teacher with a passion for clarity and precision as is evidenced by his splendid articles on crystals made from chemical melts; he was clearly a lover of flowers and his writings on those reveal a love of beauty and form; and there is his one magnificent article on mathematical forms which uses the computer program Mathematica to create astonishing mathematical oddities.
Personally, I find a combination of such sensibilities irresistible–a profound appreciation for beauty, mathematical form, and clarity. I suspect that the scientific training coupled with a unique personality had much to do with these results. And, here, I must confess to a bit of envy; my scientific training got overwhelmed by the vagaries and ambiguities of philosophy and so, to this day, I constantly struggle to be clear to myself and often fail but, at least, I am still able to recognize and appreciate clarity in others.
Let’s begin with a look at the collection of articles on crystals. From time to time, I will, and I hope with Brian’s blessing, present some of his images and comments.
The articles are extremely well organized and each begins with a description of the chemical and its properties, modes of its atomic structure, using a special graphics program called ‘HyperChem’, a comment on potential risks, usually citing the MSDS (Material Safety Data Sheets), and then proceeding to the techniques used to produce the images, followed by the images with brief commentaries. There is an exceptionally important sense in which Brian is both an explorer and a guide. He presents us with chemical crystals of substances both ordinary and specialized and manages to reveal the extraordinary characteristics of both types. Like any good guide, he knows where there are hidden dangers and provides the appropriate information and cautions without being patronizing.
He also produced a very special one-of-a-kind article. I have a B.A. in mathematics but, when I look at his splendid article on generating dazzling forms using the program Mathematica, I feel a sense of the mystical (which is an odd admission for a Spinozistic atheist). Here is a step into a whole new conceptual universe and I only wish that there had been many additional articles of this sort.
When we get to the superb series of articles dealing with flowers, we move into yet another dimension. I marvel at the way in which Brian structures his articles–my own are like rambles wandering through an uncertain landscape, never knowing when I might encounter some quicksand.
Whenever I encounter one of the flower articles, I feel like I have been invited to enter a conceptual elevator or sometimes a perceptual roller coaster. In the elevator, we start at the top with a macroview either of the plant as a whole or with the flowers. Then stage by stage, we descend and discover more and more intricate detail moving gradually to the micro levels and often down to pollen which is a mini-universe unto itself. Or in the roller coaster, we experience a series of ups and downs between the micro and macro levels providing us with a wonderfully dizzying ride.
However, let us begin with the crystals. Brian’s work is distinctive and every article carries his unique hallmark. In the crystal articles, he provides sufficient , clear information for anyone with a fair grasp of basic chemistry along with perhaps, in some cases, a dash of organic chemistry to understand the fundamentals. However, at this point, I have a confession to make–in high school and university, I hated chemistry. In high school, I had a teacher who had just gotten his M.S. and was in his first year of teaching. He gave a live lecture/demonstration in which he placed a container of water on the demonstration desk at the front of the classroom, removed a piece of metallic sodium from a container of oil with a pair of plastic tongs and dumped it into the water to show us what it would do. What it did, of course, was burst into flame and then shower the students in the front row with a mist of sodium hydroxide - that is caustic lye! From then on, I sat in the back row.
At university, I had a chemistry professor who could have cured the most egregiously severe cases of insomnia. He droned on in a monotone, reading to us from the textbook which he had written and which was also required for the course. There would have been no incentive at all to go to a lecture were it not for the fact that he had his graduate assistants keep attendance records which constituted a third of the course grade. Unfortunately, the book was as dull and uninformative as he was. What little chemistry I have managed to learn has been a solitary but, I must admit, a valuable experience, especially since I am now in my mid-70s and have not yet managed to blow myself up or poison myself. However, I am always a bit insecure with many reagents and tend, perhaps to be overly cautious. I wish I had been able to have Brian Johnston as a chemistry teacher.
One of my favorite essays on crystals is the one on Ascorbic acid (Vitamin C). Here, to my mind, Brian achieves some of his finest crystal images, but then I have a special partiality toward Ascorbic acid preparations. Some of his very best images were achieved, not only with Ascorbic acid, but with other substances as well, by making melts of the crystals by means of heating them on a slide. This technique can produce marvelous results but, it is one which I have largely avoided as a consequence of my neurosis about chemical reaction combined with my ignorance. However, in re-reading Brian’s articles on crystals in which he carefully warns against the dangers and potential pitfalls (along with urging informing oneself by reading the MSDS sheets on each chemical used and fortunately these are available online), I bought a slide warming table and have selected out a few substances to experiment with which seem relatively harmless if one takes a few simple precautions. So, once again, I thank Brian for providing me with yet another avenue of exploration in the near future. Or perhaps, I should admonish him for adding to my list of future projects which already consists of 17,231 items.
I want to show you just a few images from that article and later a few from the ones on citric acid and urea just to entice you into carefully exploring them on your own. These are especially appealing for the beginner because Ascorbic acid and urea are relatively low risk and can with a bit of practice, acquired skills, and luck produce some splendid results. So, first of all here are 3 examples from the Ascorbic acid article.

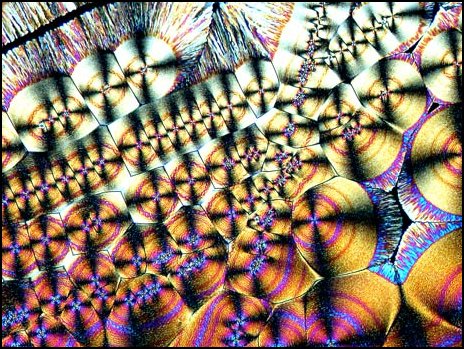
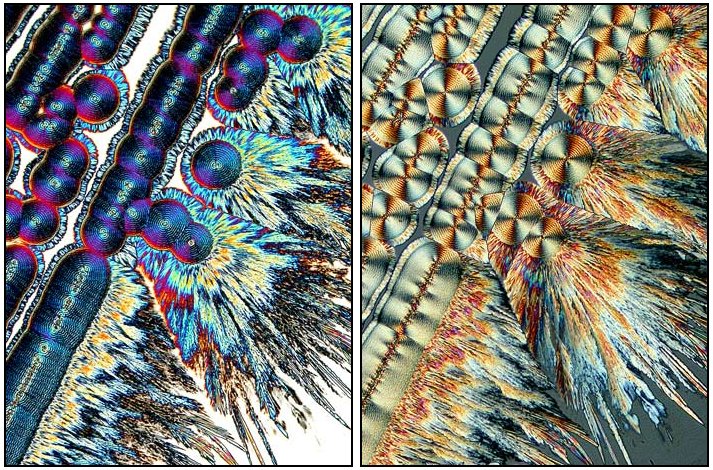
With certain reagents, Citric acid being a notable example, Brian innovates in a way which I find quite intriguing by examining some of the preparations using a phase condenser without a phase lens, then with a phase lens, in addition to polarized light and images using various compensators as well. I won’t discuss these methods in any detail for 2 reasons: 1) If I do, we’ll never even get to a paragraph examining the flower articles and 2) I don’t want to spoil your fun for experimenting and tinkering with these techniques on your own, for as Brian has noted, the slides of crystals can be frustrating in that one can try out various chemical and optical combinations and sometimes get dull and disappointing results. However, there are times when the gods smile on us and we get stunning displays such as those which Brian offers us. So, explore, tinker, innovate (carefully, of course) and open up new worlds for yourself.
Here are 6 Citric acid images showing you some of the differences.


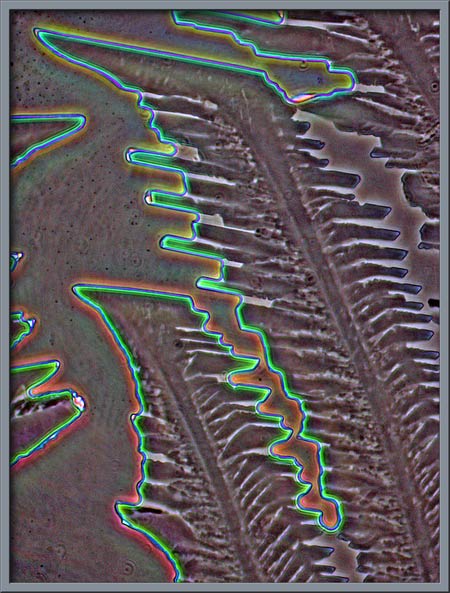



In his essay on urea, he points out that this is a good choice for a beginner to use because it is easy to work with and produces very nice results, however, one still needs to take precautions. His essay concludes with the remark:
“ Urea it seems to me, is an ideal substance to study under the polarizing microscope. It is not extremely dangerous, and once the melt specimen has been prepared, the sample can be re-melted many times to provide fascinating crystal fields. Experiment by using a pencil eraser to push gently on the centre of the coverglass while the compound is still molten. This will provide a crystal thickness gradient which will result in more interesting fields.”
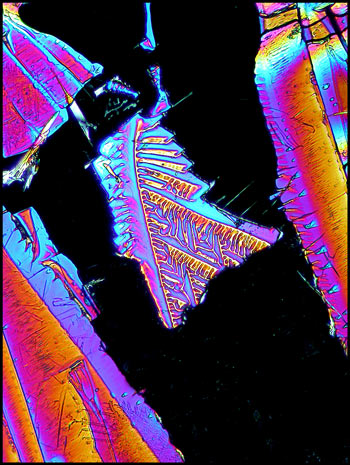
And, once again, here are 3 images to show you the wide variety of patterns.


Next, let’s take a quick look at the mathematical essay which is a display of stunning, futuristic forms. The program used here is called Mathematica and allows extraordinary possibilities for creating whole new universes of hyper-spatial images.

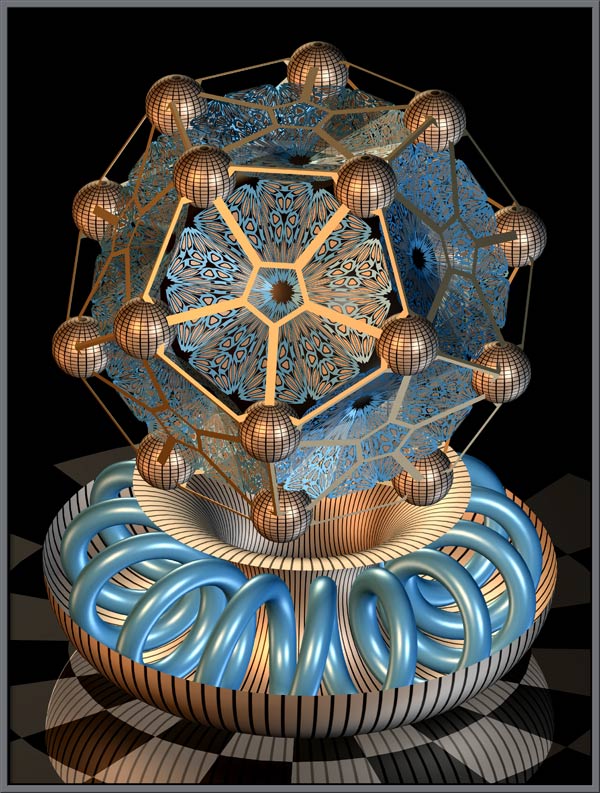


If more young students had been exposed to such marvels in their early years, they might not have developed such a strong distaste for mathematics and its powers to create entire new universes and dimensions.
Finally, we come to the remarkable flower articles about which I shall have relatively little to say since, in so many respects, they speak so eloquently for themselves. Only the most dedicated anthophobe could not love flowers. As the philosopher/novelist Iris Murdoch said:
“People from a planet without flowers would think we must be mad with joy the whole time to have such things about us.”
I am going to select a few images from just 2 of these articles which ought to be more than enough to get you addicted. The first photos come from a hybrid Blanket Flower. I’ll show you 4 images.

This is of the complete flower.

Here we see the central disk.
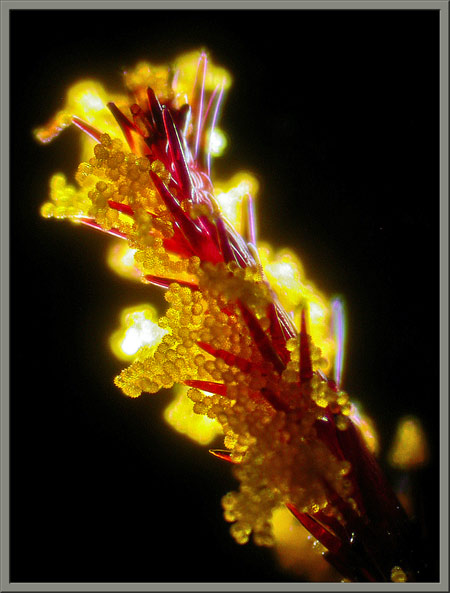
Pollen on a stigma.

And, finally, a close up of pollen on the stigma using a special lighting technique.
There are, of course, many more images of this lovely plant and by studying them, you can learn a great deal about its construction and come to a greater appreciation of its intricate structure.
Finally, let’s take a look at a few images of a very striking plant, the Passion Flower. As with the patterns on sand dollars, some people have projected elaborate religious symbolic interpretations on various parts of this plant and especially the flowers. First, a look at the extraordinary flower itself.

The combination of form and color should overcome even the fears of the most egregiously fanatical anthophobe.

Here we see some modified leaflets and sepals.

And here is a coiled tendril. Darwin was fascinated by the power of movement in plants and produced an entire book on the subject.

Finally an anther coated with pollen.
This is only the briefest overview of the contributions of Brian Johnston and I can only hope that there is enough here to encourage you to extensively explore his splendid work.
Note
All of the images in this essay are Brian Johnston’s and have not been altered in any way.
All comments to the author Richard Howey are welcomed.
All images © Brian Johnston and used with his kind permission.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Published in the July 2014 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
©
Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is at
www.microscopy-uk.org.uk .