|
Some Common Protozoa: An Oxymoron by Richard L. Howey, Wyoming, USA |
In the latter part of the 20th Century, a revolution in biological thought took place. It was a quiet revolution (except in some scientific circles) and many people are still unaware of it. The standard model that protozoa could be placed in 4 major categories was stringently challenged. Protozoa have often been regarded as primarily of interest because they were regarded as “primitive” ancestors of other life forms and the fact they occur virtually everywhere in water and soil made them seem very minor citizens of the biological community. This view was overturned and that process has had enormous implications for taxonomic or classification systems not just in terms of protozoa but, as we shall see, for our whole conception of the organization of the “tree of life”. So, in this essay, I want to look at some ways in which protozoa are quite special and then examine a few of the implications which these have for taxonomy.
From my eccentric point of view, the protozoa are all aristocrats; there are no “commoners’, although admittedly, some are more interesting than others but, I think that that view is a consequence of 1) the organism in question is so small that it’s very difficult to study and get detailed information 2) we think that they’re “common” simply because we encounter them frequently, 3) we don’t have the proper equipment to unravel their deep, dark secrets, 4) we’re appallingly lazy, 5) they don’t please us aesthetically or 6) a combination of some or all of the above. Consider the lovely coccolithophore (and no, that’s not a dirty word). I don’t have any specimens so I can’t show you any of my own images and besides these little critters are usually only about 10 microns in diameter but can produce enormous “blooms” in the northern seas such that satellites can detect them and they have marvelously elegant calcareous shells which Scanning Electron Images reveal to the delight of the eye and the psyche.
So, what I propose that we do is take a brief journey and look at some very special features of “common” protozoa. Let’s start with the ubiquitous Paramecium because, if you took a biology class that had a microscope and didn’t ever look at a Paramecium, then it was a lazy, lousy science teacher unless it was taught in an area of sub-Saharan Africa where there were no oases.
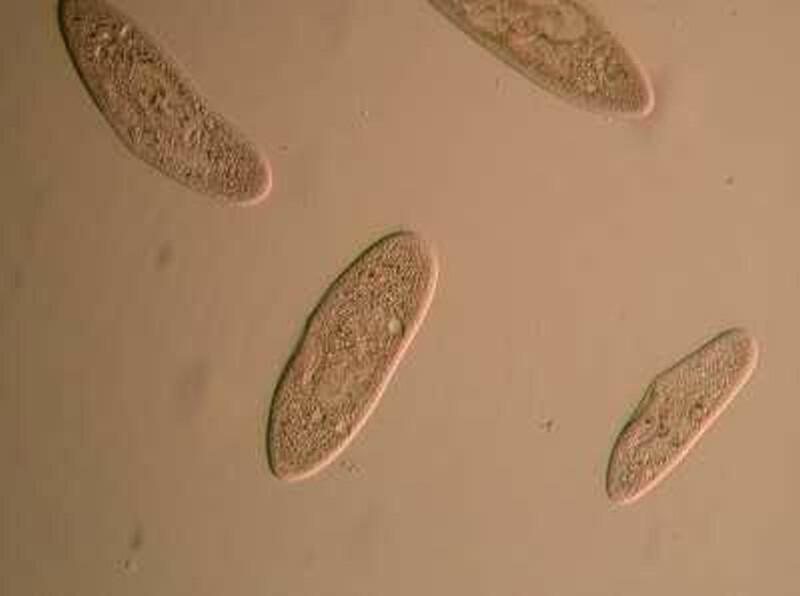
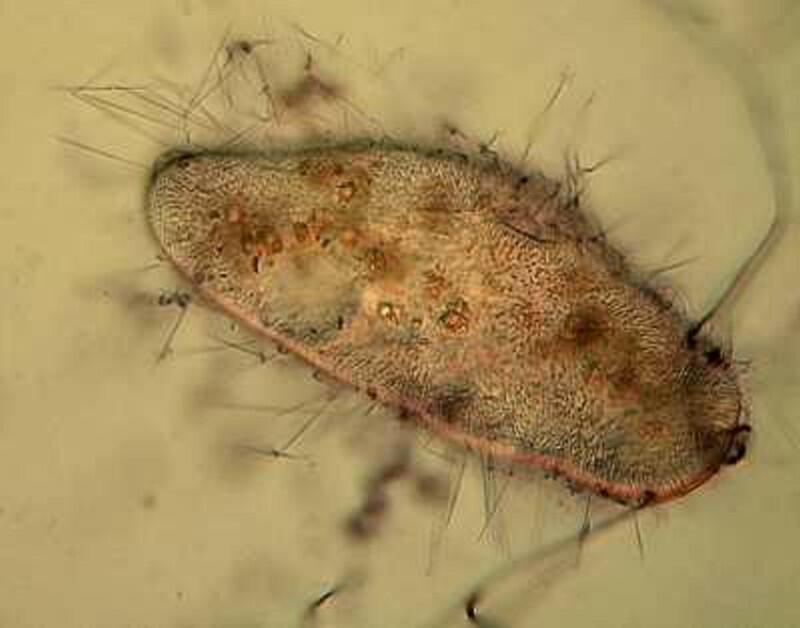
Here are some little slipper-shaped creatures from one of my pond cultures.

If this were the only view you ever got of them, they might seem very uninteresting indeed. However let’s do a bit of a high-tech jump to a Nomarski Differential Interference Contrast image at 400 magnifications.
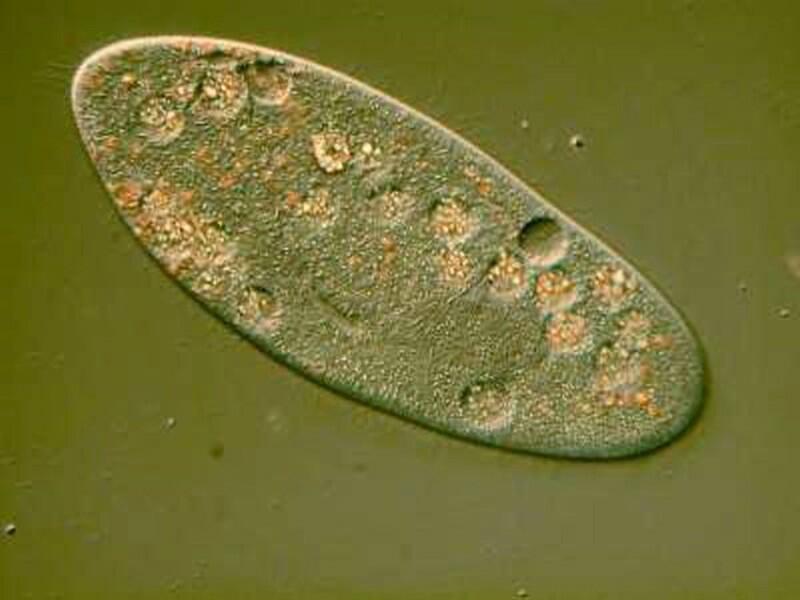
Here we can see some structures that we would never have guessed at from the first image.
TANGENT. Please feel free to ignore this part and forever suffer the curse of Thoth. If one is attuned to the affinities of the sciences and the arts, then one quickly comes to realize that often vibrant creativity depends upon having fine, and unfortunately, expensive equipment. We should constantly encourage curiosity and talent in the young, but that is extremely difficult to do if the best you can offer to a child who loves sound is a $10 violin or a youth enraptured by the microscope a $25 instrument with plastic lenses and such severe spherical and chromatic aberration as to make whatever is visible, a distorted caricature of how it should appear. This of course, doesn’t mean that one has to start with a Stradivarius or a Zeiss. However, one should try to find a good used instrument with fine lenses and a brand that still has a good range of accessories available.
END OF TANGENT.
If we go back to the DIC image of the Paramecium, certain features are immediately obvious: 1) one can clearly see the hair-like cilia along the edge of the membrane of the organism. Here, we need to remember that DIC produces very thin views of “sections” and so employs a technique which is described as “optical sectioning”. This means that as you make even very small adjustments with fine focus, the shift in the focal plane will provide you a new perspective and often new detail within obvious limits of the focal depth which the organism (or specimen) allows. In other words, this technique allows you a series of optical “slices” which you can use either photographically, through drawings, or your own visual memory to construct a more comprehensive and detailed image. The cilia of the Paramecium here is one case in point for, in looking at the image, we may very well naturally jump to the conclusion that cilia occur only around the edges of the organism whereas, in fact, this organism has no “edge” in that sense; it is a 3-dimensional creature with cilia distributed over its entire surface and, if one is careful in adjusting the focal plane using DIC, it is possible to get a nice view of the cilia along the top of the membrane.
In fact, if you look very carefully at this image, you can see some examples, but here they tend to look flattened out rather than projecting upward which is a result of the pressure of the cover glass.
Just slightly off center is a sizeable circular structure containing several tiny orange spheroids–this larger structure is the macronucleus.
You will also notice more than a dozen smaller spherical structures filled with tiny colored particles; these are food vacuoles. These appear to be more or less flat to the surface of the membrane or slightly raised with one exception which seems empty of content.
Returning to the present image, if you look closely along the edge, just inside the membrane, you notice minute structures that look a bit like elongated isosceles triangles. These are the “capsules” containing the mysterious trichocysts. You might think of a trichocyst as a tiny harpoon attached to a long, coiled segmented rope waiting for some physical or chemical stimulus to cause it to discharge which they can do with relatively enormous force as is evident from the 4 images below.
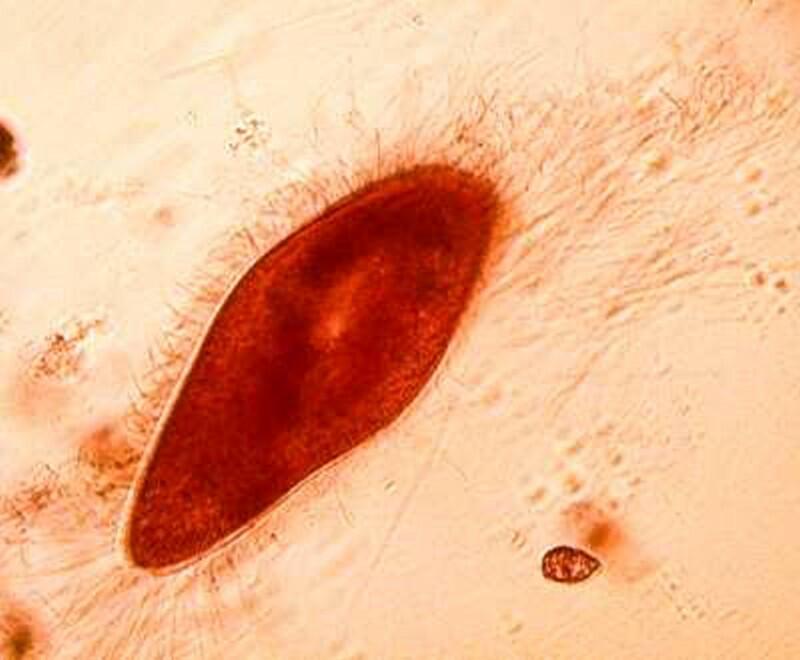
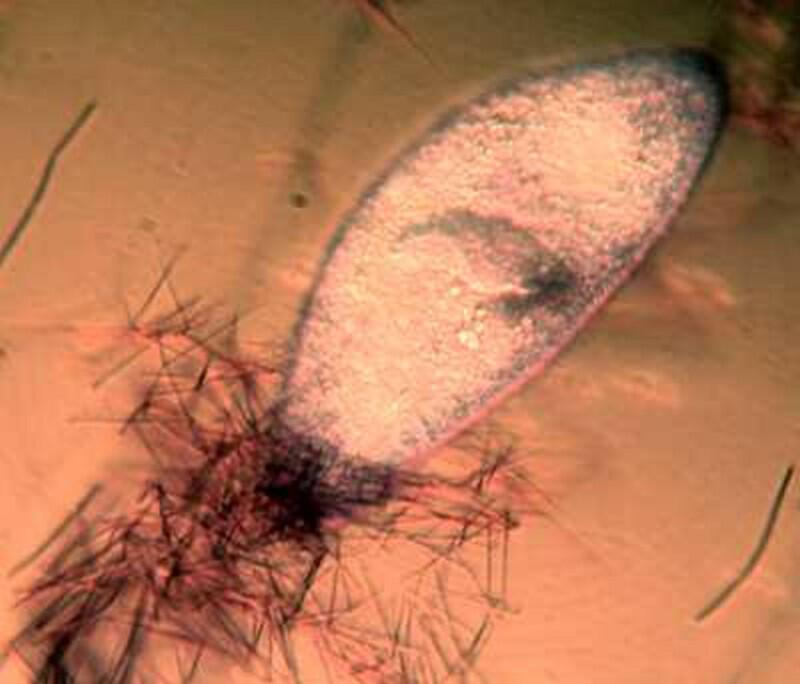
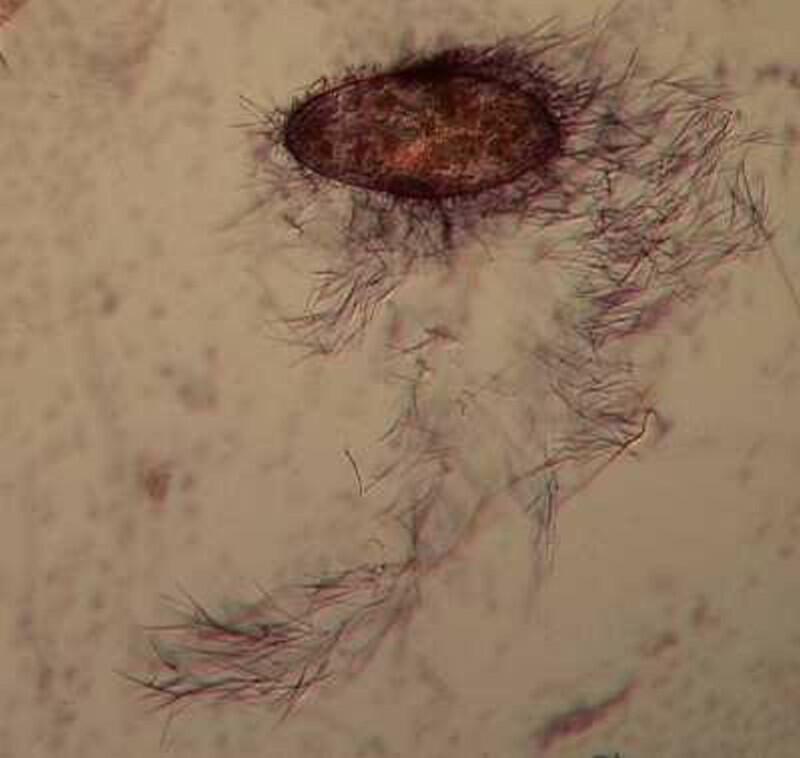
As you can see, these “barbs” can be ejected several times the length of the body of the Paramecium. Imagine the energy involved in this process! It is as though we had thousands of tiny torpedoes in this micro-submarine just waiting for a signal to launch–an enormously, heavily armed micro-critter constant on the ready to loose its array of formidable weapons, but to what end? Imagine such a potentially formidable arsenal being fired off and hitting (but not disabling or deterring a single enemy), then one has to ask: Why aren’t these super-weapons effective? (Of course, we might think of the United States infamously ineffective, flawed, and outrageously expensive “Star Wars” project.) Trichocysts seem to be notoriously ineffective. Their function has been debated for over a century and a half: 1) They don’t seem to prevent the Paramecium from becoming a tasty meal for a larger protozoan, 2) Their targeting system certainly doesn’t seem to be very efficient; in other words, large numbers of them seem to just fire off randomly once there is a trigger stimulus. 3) It is barely conceivable that they were designed to created a small protective “net” but, at best, this could discourage only other quite small protozoa which would most likely be potential food rather than posing any threat. 4) Just to make things more interesting, there are other protozoa which have a modified version of these structures which possess a droplet of toxin at the tip and can be used for stunning prey. These structures are appropriately called toxicysts and in the large ciliate, Dileptus, these weapons are arranged around the cytostome, an energy-efficient system. In many coelenterates, including the freshwater Hydra, we can discover such “stinging cells” which have the same purpose. In the earlier days of my ignorance and naivety (I have now progressed to my later days of both), I blithely assumed that there was a single basic type of “stinging cell” or nematocyst only to discover that in Hydra alone, there are at least four different types. (Obviously a topic for another whole essay.)
Again back to a Paramecium DIC image but this is a slightly different view at 630 magnifications.
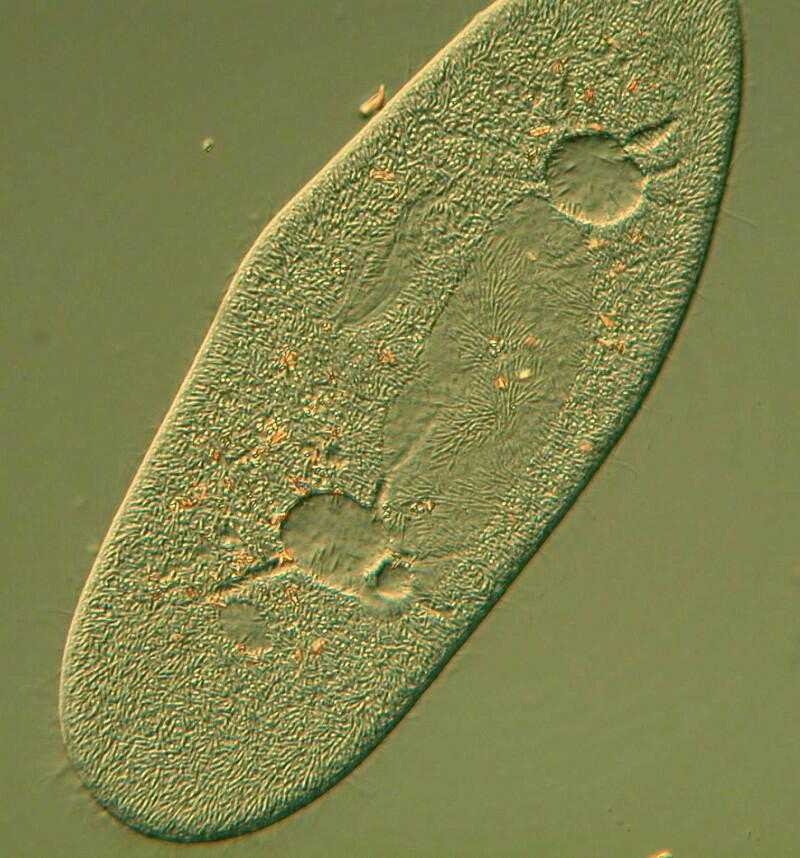
A number of major features jump out at us. The macronucleus in this species is distinctive as are the contractile vacuoles which in this organism are incredible structures. When free-living protozoa feed, significant quantities of water enter along with the prey organisms. This creates an imbalance between the osmotic pressure inside the organism relative to that of its external environment. If there were no mechanism for restoring a balance, this would soon destroy the Paramecium. Excess fluid accumulates in these vacuoles and when the pressure is sufficient, it is expelled back out into the surrounding environment. In Paramecium this process is especially intriguing. In many protozoa, the contractile vacuole is often a simple expandable spheroid or “bubble”. However in Paramecium not only is there such a spheroid, but a series of “canals” which collect excess fluid and channel it to the central spheroid. This small complex looks like a miniature flower and when the central spheroid reaches a certain volume it ejects fluid through a cytopyge (a minute opening in the membrane).
This image provides us with some additional cytological information. 1) The majority of the surface does indeed look hairy indicating the very large number of cilia in the Paramecium membrane. 2) We can see a large, elongated mass which is clearly well within the organism since we can observe cilia above it; this is again the macronucleus and has this rather exaggerated elongation as a result of the pressure of the cover glass. 3) Just above it and below it, we notice 2 spheroids which seem to project upward and have a few oddly triangular processes which extend into them. The number is usually 5 or 6 and these are the canals that siphon excess fluid into the vacuoles to be expelled through the cytopyge thus helping to restore the osmotic balance. Just to the right of the flattened macronuclear mass is an odd (and not very well defined) structure which is a part of the canal which leads into the cytostome (mouth).
If we return to the first image
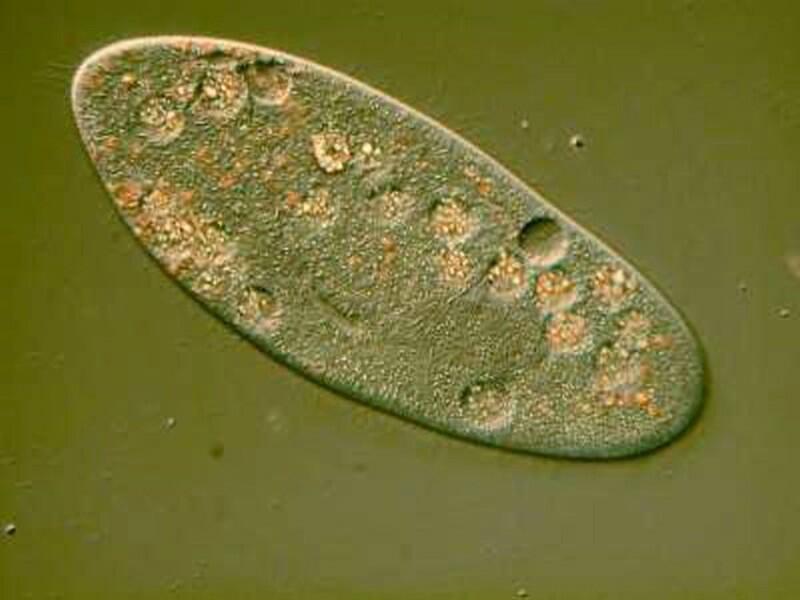
we can see lots of little granules. In some instances they occur in clumps and are birefringent so that when we observe them live under polarized light, we see a miniature swimming light festival.
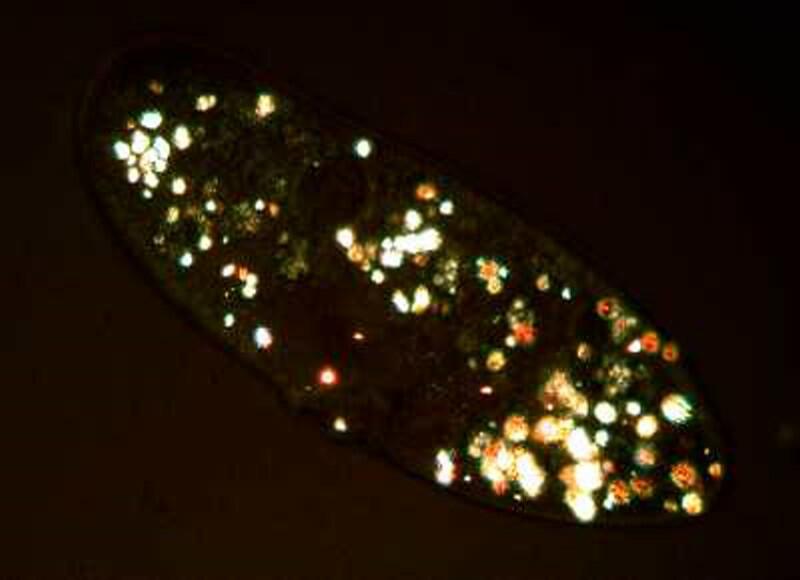
So, the next time someone tells you that a Paramecium is just a common ordinary critter, you have some strong reasons for objecting.
Sadly, another group of protozoa that is often given only passing attention is the amoebae who, in spite of their reputation as “blobs” or “bits of protoplasmic jelly” do, in fact, show remarkable variation given their general body plan”. They range in size from the giant Pelomyxa which can be over 5000 microns to species that are less than 10 microns. There are even a few species in which there are stages in which they possess flagella!–Mother Nature throwing us another curve ball. Again, there have been debates as to whether or not Pelomyxa is really an amoeba in the traditional sense or whether it belongs in its own separate taxonomic niche due to some very odd characteristics. There are a few types of amoebae which possess a knobby lump-like structure at one end–the end away from which the protoplasm is streaming. This structure is called a uropod and seems to act as a kind of moving anchor. In addition, there are finger-like processes which extend and retract in other places on the surface of the membrane. The function of these is poorly understood. Furthermore, Pelomyxa forms one large pseudopodium unlike the class Amoeba proteus which sends out a number of pseudopodia in a variety of directions engaging in what might be described as a search maneuver for food.
Flagellated amoebae pose another set of problems for taxonomists and here we move into levels of very deep weirdness. It has been argued that flagellates are probably the most “basic”, “fundamental”, “foundational” of the protozoa, because of the fact that they possess flagella. Well, you already guessed that it wasn’t going to be that simple. The argument hinges on claims that pose some major morphological challenges. Mother Nature just loves to torment us by introducing staggeringly difficult subtleties–isn’t it wonderful?!
It has long been known that there are certain types of bacteria which possess one or more flagella and here we run into a major stumbling block–bacteria are prokaryotes and protozoa are eukaryotes–this is a radical difference because prokaryotes don’t have nuclei where as eukaryotes do. Now, in the late 20th Century, this got some biologists deeply taxonomically and terminologically agitated. A key figure was the distinguished biologist, Lynn Margulis, who was adamant that the term “flagellum” be applied only to prokaryotes and that the term “undulipodium” be used to describe the “thingy” that we have for so long been accustomed to designate as a flagellum. So, what’s all the fuss about? As it turns out, there are some quite intriguing problems embedded here.
As is well known, we are hosts to other organisms; parasites, benign viruses and bacteria as well as pathogenic forms, and most importantly, beneficial ones without which we couldn’t survive. But beyond that,
“We are shared, rented occupied. At the interior of our cells, driving them, providing the oxidative energy that sends us out for the improvement of each shining day, are the mitochondria, and in a strict sense they are not ours. They turn out to be little separate creatures, the colonial posterity of migrant prokaryotes, probably primitive bacteria that swam into ancestral precursors of our eukaryotic cells and stayed there. Ever since, they have maintained themselves and their ways, replicating in their own fashion, privately, with their own DNA and RNA quite different from ours. They are as much symbionts as the rhizobial bacteria in the roots of beans. Without them, we would not move a muscle, drum a finger, think a thought.” (Lewis Thomas, The Lives of a Cell, Penguin Books, 1984, p. 4).
There are other theories which propose even more surprising notions regarding the profound evolutionary dependence which our existence has on peculiar alliances between primitive bits of living material which barely reach the minimum standard for being organisms.
“It has been suggested that flagella and cilia were once spirochetes that joined up with other prokaryotes when nucleated cells were being pieced together.” (Lewis Thomas, op. cit., pp. 70-71)
These notions should give us a profoundly different view of ourselves as organisms and also radically alter our conception of the evolution of complex organisms.
Some biologists argue (most stringently, Lynn Margulis) that the term “flagellum” should be applied only to the prokaryote structures since they actually rotate and there is a tiny, unique “motor” at the base of each fibril which is responsible for this. What we have traditionally called a flagellum in eukaryote protists doesn’t rotate, but undulates which leads Margulis to argue rather vociferously that what we used to call flagella, now be called undulipodia. The derivation is obvious but, nonetheless, it is a rather ugly word and most other microbiologists (the distinguished protozoologist, John Corliss among them) have ignored this urging and persist in using the term “flagellum” in its traditional way, even though they acknowledge the difference between the 2 structures. Scientists, like philosophers, are vulnerable to nitpicking. Perhaps the easiest resolution might be to call the traditional eukaryotic structure flagellum 1 and the prokaryotic structure flagellum 2.
Well, anyway, back to the flagellated (undulipodiated) amoebae. How the traditional flagellum evolved is a highly complicated and not well understood issue and this is an organelle of great significance in the process of evolution. Among this highly diverse group of flagellates, we find “collared” flagellates or choanocytes and forms of these are major components in sponges which are colonial organisms and, just to make things even more splendidly complicated, also have amoebocytes.
So, there are many biologists who regard flagellated protozoa as a transition to sponges. However, yet another difficulty arises because, sponges, interestingly, are an evolutionary dead-end. This is a tricky concept but, basically, it means that while there are still plenty of sponges around–they are very good at adapting and surviving–this phylum hasn’t led to the evolution of any other organisms “higher” up the evolutionary tree. In other words, sponges don’t seem to be a transition to any other group of organisms.
For a long time, amoebae were thought to be the most “primitive” of the protozoa, but that view has been re-examined and is no longer part of the dogmatic textbook lore. I also question the whole idea of there being a phylum called Protozoa, since historically, its membership was based on a concept of the members constituting it being unicellular. I have argued elsewhere that the organisms in this phylum are complete, distinct individuals and not cells.
If we ask the question” What do naked amoebae, radiolaria, sporozoa, flagellates, ciliates, and suctoria have in common that justifies our placing them in the same phylum, the answer is virtually nothing, except the wrong-headed claim that they are unicellular.
A different way of approaching this issue is from the opposite direction, namely, noticing that all other traditional phyla consist of organisms which are composed of cells so, at first gasp, it appears perfectly reasonable that all those eukaryotes which aren’t composed of a variety of types of cells are thus “unicellular”. This is a colossal mistake because protozoans are complete, total independent organisms and are quite unlike the cells that compose tissue whether it be muscle, gland, lung, kidney, or brain. This, lumping together of ciliates, amoebae, sporozoans, and flagellates is not justified simply on the basis that they are not cellular.
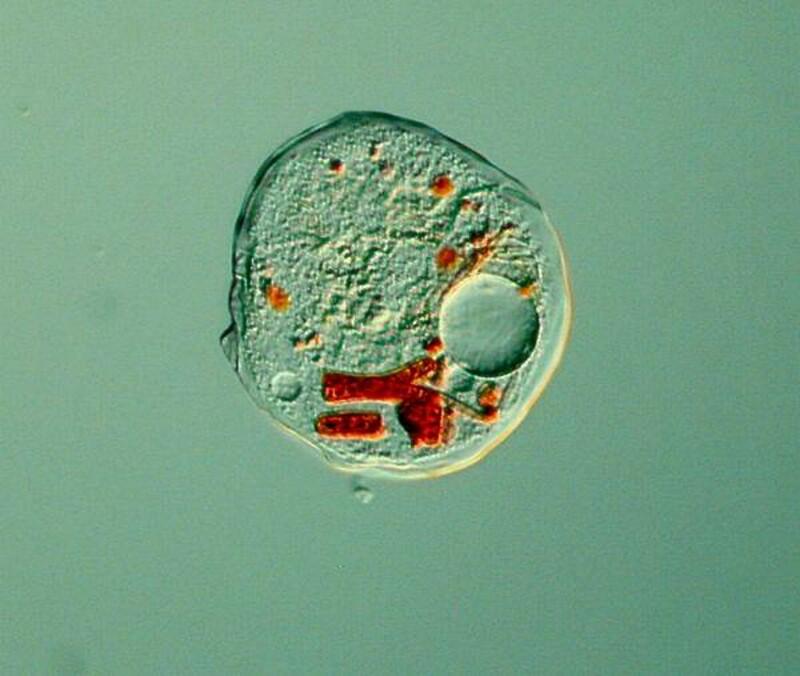
An intriguing aspect of amoebae is that they are shape-shifters–however, not in the dramatic manner of Odo in “Deep Space Nine–in that when they are active, we can watch them move and later alter form, sometimes in quite striking ways. As we watch, we are even further impressed by the fact that these “brainless” organisms can sense prey and shift their protoplasmic flow to surround and capture that prey. They do have resting stages and most of them are voracious feeders. Take a look at this ambitious little amoeba in the process of ingesting an algal filament larger than it is.
Thecamoeba is also an algae eater and guilty of the deadly sin of gluttony according to the Supreme God of Amoebae. I stained some algal filaments with a non-toxic vital stain to get a better sense of their feeding pattern and you can see the results below.
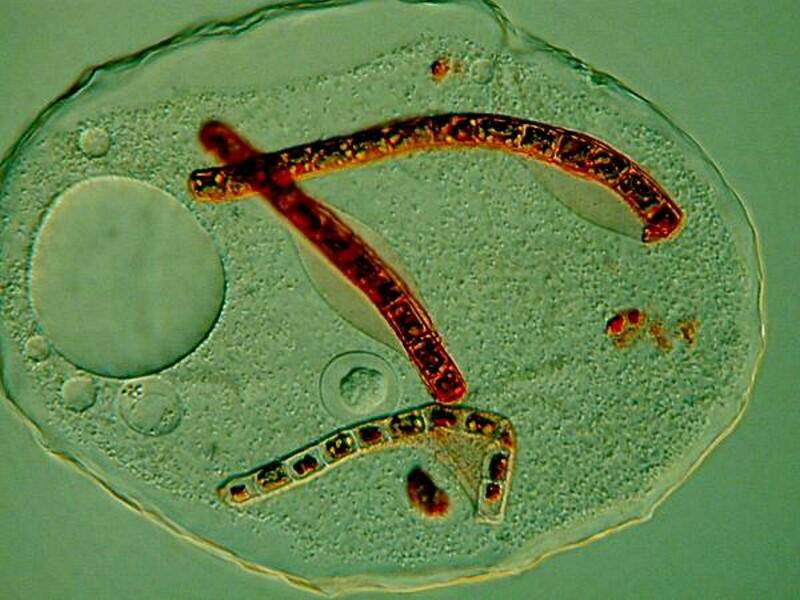
This intriguing organism has a wrinkled membrane and I have called it the Shar-Pei of amoebae. If you want to know bit more about them, you can glance at an article which I wrote earlier for Micscape.
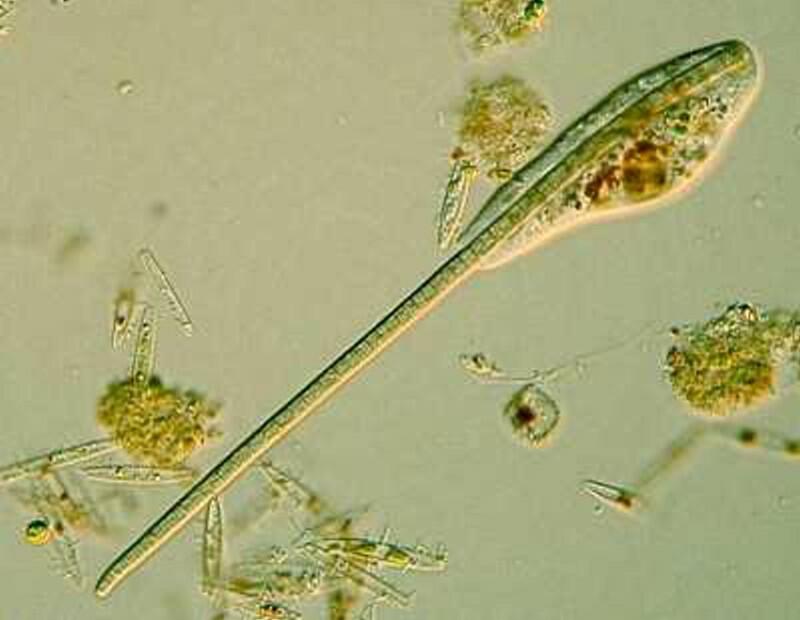
Another organism frequently encountered in ponds is Euglena and although it is common, it by no means ordinary. Below is an image of Euglena acus.
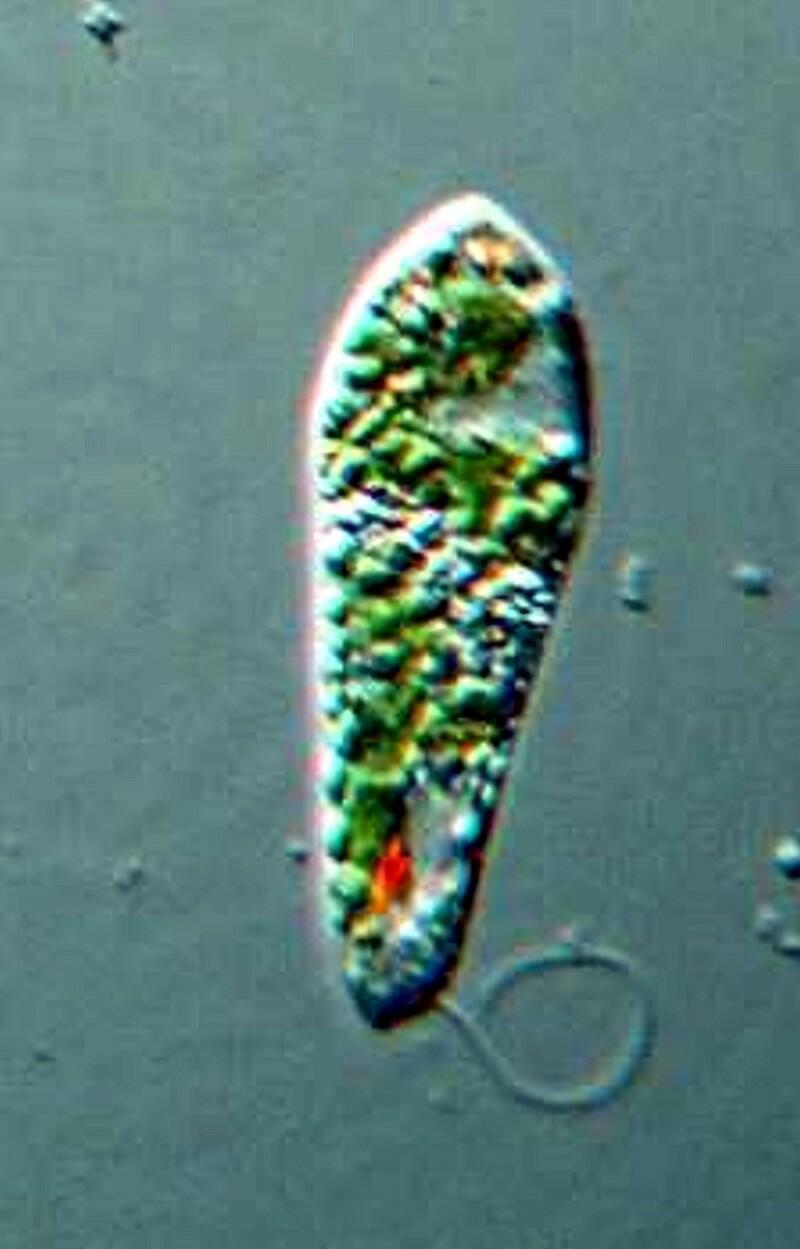
As you can see it has a flagellum, a bright red eye-spot which filters out certain wavelengths of light which then reach the paraflagellar body and this process helps the organism orient itself in relation to light. The eye-spot stands out against the overall green background which is due to the presence of chloroplasts which provide food by means of their photosynthetic activity.
I have mentioned 3 of the 4 traditional groups of protozoa and the fourth is the Sporozoa, all of which are parasitic, often have complex life cycles, and in the mature stages are non-motile.
Now, the major issue becomes: What do these 4 groups have in common? and the answer is–not much, except that they are NOT composed of cells (which again, is no justification for describing them as unicellular!) There is a strong morphological correlation between the ultrastructure of flagella (undulipodia) and cilia but, this is also the case between the cilia of a Paramecium and the cilia in the human body. As we noted above, there are a few species of amoebae that, at least in certain stages, possess a flagellum or perhaps several. Does this mean that they are transitional forms? Does it mean that they belong in a separate taxonomic unit? What is clear is that more and more taxonomy is based upon the results of information derived from SEM, TEM, and laser confocal microscopy, molecular biology, and genetic sequencing. This is not to say that morphological analyses derived from optical microscopy are irrelevant but, they’re certainly no longer determinative. Where does this leave the amateur beyond the obvious state of confusion? Since the middle of the 20th Century, very few amateurs have gotten embroiled in the taxing issues of taxonomy. With the rapid development of new kinds of microscopes and new types of microtechnique beyond the reach of amateurs, ultrastructure has become taxonomically determinative.
I can’t claim credit for any of these revolutionary ideas; you’ll have to blame Margulis, Corliss, Melkonian, Chapman, et. al. for their infamous Handbook of Protoctista, although as you shall see, I am perhaps more radical in one or two respects. When this tome came out in 1990, I paid $80 for it which I considered a bit of an extravagance, but it is 915 pages, printed on high gloss paper, well-bound, and filled with optical, TEM, and SEM photomicrographs so, from my point of view, it was a good investment. Today, you can pick up a used copy for $199.00, $199.95, $335.84, $335.87, $369.51, or a special bargain price of $4,999.00 (I’m not kidding; there is a dealer who has such a listing!) Or, if you’re not in a hurry, there is a second edition in the works which has 950 pages and will list at $399.95 and will be discounted by Amazon.com to $345.28. As some of you know from previous essays of mine, book prices are a special pet peeve which I rant about from time to time.
A major thesis of this work which a number of modern biologists are proposing involves the 5 Kingdom model which says we can no longer think in terms of the simple (and simplistic) biological model of dividing all life forms into 2 Supercategories: Plants and Animals. The 5 Kingdoms are Plants, Animals, Fungi, Monera, and Protoctista. However, some are arguing that there should be a 6th Kingdom. My own view is that we need many more than 5 or 6 Kingdoms. I would also note that “Protoctista” is not a felicitous term and many scientists have ignored it and returned to the older and simpler term “Protista” which Haeckel devised.
There was a period in the 19th and early 20th Centuries when the category du jour was “Infusoria”. This designation could include ciliates, flagellates, amoebae, desmids, diatoms, rotifers, algae, rotifers, and sometimes other small aquatic invertebrates. In other words, it was a sort of catch-all, the composition of which often depended upon the bias of a particular researcher. Well, in my view, “Protoctista” is not all that different; it is a catch-all and mishmash of something like 36 or 97 phyla. I counted once and I don’t remember the exact number so, if you want to know, you’ll have to start counting. The problems and questions which this arrangement poses are enormous. For example, what do giant kelp have in common with amoebae, slime molds with radiolaria, or Volvox with sporozoa that would induce one to put them in the same Kingdom? And, if you think that “Protoctista” is a terminological cicatrice, then you’ll love the proposal that we now refer to the Foraminifera (our old friends–the forams) as Granuloreticulosa.
Despite the creation of a series of linguistic carcinomas, this new scheme/model does have the virtue of going beyond the old phylum designation of Protozoa and recognizing that there is a series of diverse groups that require separate categories. Under the 5 Kingdom mode, Protoctista has a separate phylum for the euglenoids; a separate phylum for ciliates, a separate phylum for amoebae, but separates out Pelomyxa into a separate phylum (Karyoblastea), and also separates Heliozoa, Acantharians, and Radiolaria into a distinct phylum and, as well, Dinoflagellates now have their own phylum.
Conceptually, this is a kind of progress, since it forces us to recognize that we need to make more precise distinctions between these various organisms. In the long run, this will serve to stimulate yet further analysis regarding the similarities and differences regarding these various groups of organisms. In the short term, this new approach creates confusion, resistance, and even antagonism. This can be good, if its critics are thoughtful and insightful. Coccolithophores now belong to a phylum with an even stranger name–Prymnesiophyta. (Not exactly a name for a cheerleading chant–Give me “P”, Give me an “R”, Give me a “Y”, etc.)
Hang in there, I’m getting near the end (for now). While I see this approach as significant progress, I still have a major problem about lumping an enormous number of highly diverse organisms into this single Kingdom, be it Protoctista or Protista. Nonetheless, this model does have the virtue of substantially breaking down the old notion that Protozoa can be placed in a single phylum on the basis that they are “unicellular organisms” (oxymoron alert!)
However, I am still not convinced that putting giant kelp, Amoeba proteus, radiolaria, ciliates, and dinoflagellates all in one Kingdom is very helpful in the long run but, that is a for a future discussion.
All comments to the author Richard Howey are welcomed.
Editor's note: Visit Richard Howey's new website at http://rhowey.googlepages.com/home where he plans to share aspects of his wide interests.
Microscopy UK Front
Page
Micscape
Magazine
Article
Library
Published in the November 2012 edition of Micscape Magazine.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK website at Microscopy-UK .
© Onview.net Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights reserved. Main site is at www.microscopy-uk.org.uk .