Issue:
I have a lot of fun collecting and researching daphnia, and over time have found that it doesn't take a lot of effort or expense. While I have found a good number of technical articles on daphnia, much of what I have learned on an amateur level has come more by experimenting and making mistakes, than from any written material. I would like to share some of these ideas, to encourage others along similar activities, and possibly stimulate discussion and discovery of other techniques.Because daphnia are used in water
quality testing, there are published standards for cultivating them; but
these consist of formulas for making up culture water of known chemical
composition, and other techniques that are more work and expense than I
can get into. If you are not out for scientific quantitative analysis,
much of the discipline can be dispensed with. While I would love to have
a boat and Birge nets for deep water sampling, there is a lot that can
be done for very little cost. Outside of my microscope and camera, I have
probably spent less than $200 (US) for everything else I have obtained
over the last several years; and the largest portion of that has probably
been for hand lenses, slides and supplies used to make permanent mounts
(this is several hundred slides).
The Equipment Basics:
Something to collect with:
The most useful tool I have encountered is a "Turkey Baster", one of those plastic tubes with a rubber bulb on the end, which are sold in grocery stores for basting meat. Dipping a bottle in a pond can be rather hit-or-miss on the results (but always better than nothing). The baster lets me target individual moving critters, as well as exploratory sampling under rocks or in vegetation.
Something to put them in:
Any container that holds water will work, though I am finding the 1/2 liter water bottle the most useful. They are small enough to carry around, yet hold enough to be a good sample; besides they are easy to obtain. On a recent trip to Lake Michigan, I found enough bottles to keep me happy just picking up the discards along the beach. Other people may have thought I had good intentions of picking up litter, but my family knew my real motives. To avoid surprises, be sure to label what is your drinking bottle and what is your collecting bottle.I have used clear 2 liter soda bottles (and still like them for culturing daphnia), but find them too big for carrying around. They are also more of a nuisance, as they have to be well rinsed to remove the traces of sugar and chemical residue of the soft drink. Bottled water advertises that it doesn't have chemical additives!
Plastic disposable food containers
are convenient for collecting plant samples, though they run more risk
of popping open when full of water. For safety, put them in another universal
tool - the zip-lock plastic bag. In addition to daphnia, I like collecting
hydra. I have never spotted a hydra in the wild, but collecting plant material
in daphnia habitat often brings results. Collect plants and sticks, and
let them sit for several days; the hydra can be spotted on the plants,
or more likely clinging to the side of the container.
Culturing Daphnia:

Two liter soda bottles make excellent small aquariums. Take one bottle and cut off the top about an inch above the 'shoulder', and cut another one at the shoulder. You now have a nice 2 liter aquarium with cover.
In addition to these I have a collection of smaller bottles and jars for isolating individual specimens, or for starting a 'pure' culture (i.e. trying to separate one daphnia species from a bunch of ostracods or copepods). While I have tried culturing 'green water' so to feed the daphnia algae, it is more work than it is worth (in my opinion). The easiest thing is to feed them yeast. I put 1/2 teaspoon of bakers yeast in a 35mm film container filled with water. One drop of this solution per 2 liters, every day or two, keeps most of my cultures in a healthy state. This may not be the best means for mass production (if you are trying to raise fish food), but does keep them alive and healthy. I always keep at least two containers of any species, as inevitably a culture will eutrify over time and 'crash', killing all the inhabitants. Usually, before they reach that point I will split a culture into new ones. It may be my lack of 'sterile culture', but I usually find ostracods taking over my cultures before they are old enough to crash.
I tend to keep my cultures under
lights for 8-10 hours a day, but the daphnia and hydra will live quite
happily without it. The lights are more for the plants and algae in the
bottles than required for the daphnia.
Observations:
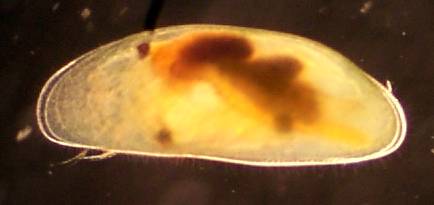
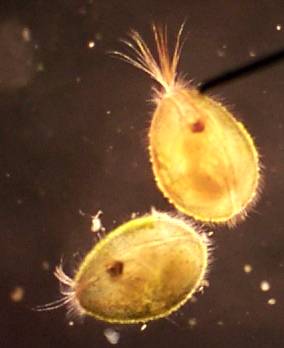
One of my favorite sites has been Grasso Spring in south St. Louis. It is located on a bike path I like to ride. It reliably has ceriodaphnia, and the last time there (April 27, 2001) I found some green hydra. There is a 2 foot deep spot near an outlet drain that usually produces a good sample with a little hunting. It has grown over with cattails in the last year, but is still a good site.


View of Grasso Spring from the TrailNet parking lot
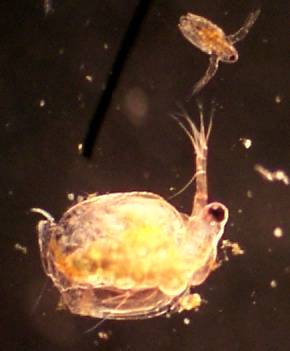
Ceriodaphnia 100x, 2x digital 'good' quality |

Green Hydra 8x, 2x digital 'good' quailty |