|
PART 1
There are included here many pictures published by the kindness of the authors, to which I offer my most sincere thanks. Especially to M. Verolet, whose generosity has provided many of the most interesting pictures shown here. I have also used drawings sourced from some Internet websites for which I give the required notice. The remaining illustrations were taken (in these times of digital cameras with 5 to 10 Mpx) with a camera of 0.4 Mpx; certainly they have considerable work-up using PhotoPaint, NetImage Demo, and ACDSee.
I hope that, at least, they will be clear and convey the information that they are intended to give.
INTRODUCTION
In traditional taxonomy rotifers are considered a phylum which embraces three classes: the "Seisonacea", the "Monogononta", and the "Bdelloidea"
(see Taxonomy footnote). Seisonacea
are solely marine epizoics on Nebalia, (a genus of crustaceans considered very primitive, benthic, with some litoral species), and are represented by only one genus, Seison, with three species, the last one described in 2007, which have a morphology very distant from that normally assigned to a rotifer. Monogononta, thus called because male and female have only one genital gland, were extensively discussed by Michele Verolet, who has presented in the French Magazine Microscopies a very complete and heavily illustrated description of their morphology, and a splendid key to identify all the genera that the amateur has the possibility of finding with some frequency in their explorations.
http://www.microscopies.com/DOSSIERS/Magazine/Articles/M%20Verolet-CLE/presentation/presentation.htm
Bdelloidea
are all parthenogenetic females, with two genital glands. (For a long time they were called Digononta).
Monogononta and Seisonacea have males but Bdelloidea haven't had them ....... probably for 80 million years
(http://news.bbc.co.uk/1/hi/sci/tech/7039478.htm)
Their reproduction is exclusively parthenogenetic, and the geneticists struggle to discover the mechanisms which made it possible for these animals to maintain their genetic diversity and to avoid being banned from the list of living species. Some recent papers show that they steal genes from other species, to modify their own genome.
Of all the rotifers the Bdelloidea are the best known by the non-specialized worker. The anterior end has a "corona" divided into two retractile "trochal discs", with ciliated margins. The cilia beat rythmically, and seem to rotate, which gave the name to these animals. In fact the trochal
discs are restricted to 15 of the 19 genus of this Class, but the
ciliated corona are so showy that the feature gives its name to the Phylum Rotifera.
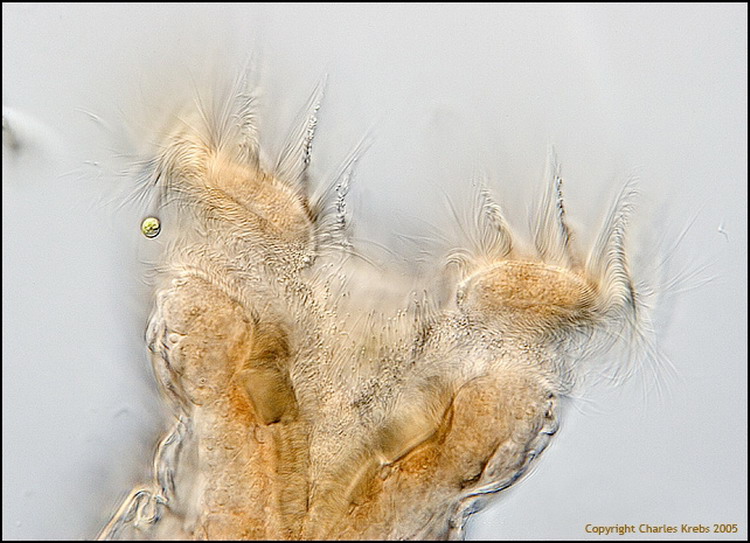
2 - Magnificent frontal view of the "head" of a Philodina (courtesy of Charles Krebs).
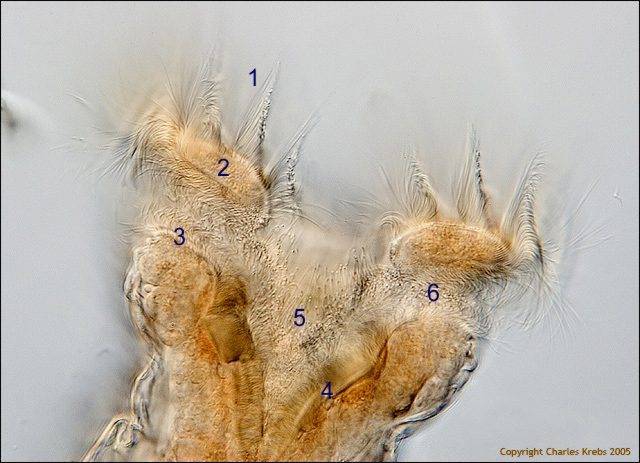
2a - Annotations by the author: 1 - lines of cilia implanted in the trochal disc
border (2), 3 - inferior lips or cingulum, 4 - lip of the buccal funnel, 5 - buccal funnel, 6 - sulcus (a furrow between trocha and cingulum). These ciliated features help to send the food to the mouth.
They are multicellular organisms with a fairly complex organization, but are similar in size to that of the large protozoans, (between 150 and 700 microns). Only one species, Rotaria neptunia, is excessively long and thin, reaching a size of 1600 microns. (Ricci and Melone, 2000.)
Practically all the species can endure drying and can revive when they happen to be resubmerged in water.
Jacobs published in 1909, for the first time, the description of the amazing capacity of these rotifers, which gives them the possibility of invading really difficult habitats, like desiccable mosses, the cracks in the bark of trees, the soil, and so on.
The rotiferologist Aydin Örstan was able to find bdelloids in the wall of the tubes of termites, adhered to a tree in Puerto Rico, and even described a new species from the dusty soil of the
Sonoran desert in Mexico (Macrotrachela sonorensis).
Christian Colin recorded the images of the drying process and the revival of a bdelloid. The article that describes his technique, process, and results can be read at:
http ://www.microscopies.com/DOSSIERS/Magazine/Articles/CC-PHENIX/Phenix-1.htm
and its second part
http ://www.microscopies.com/DOSSIERS/Magazine/Articles/CC-PHENIX/Phenix-2.htm
another shorter graphic document on the same phenomenon written by Hugo Baillie-Johnson can be found at
http://www.microscopy-uk.org.uk/mag/artnov02/hbjtrehalose.html
3 - Revival, after 46 days of anhydrobiosis. Recorded by Christian Colin.
In the state of anhydrobiosis (or anabiosis) they resemble small dust particles and, like them, they can be dispersed easily by the wind, or the waters that bathe the surfaces where they live. Notwithstanding, there are in fact few recorded studies on the real (and surely multiple) dispersal
methods that the rotifers use, and which have given to many species the ability for them to become cosmopolitan.
The anydrobiosis is not a simple adaptation to colonize difficult
habitats. As Ricci et al states (August 2007):
"Bdelloids, although aquatic animals, are not only efficient in tolerating desiccation, but seem somehow dependent on anhydrobiosis, a circumstance that might represent a key event in their life cycle. If this is true, life in unpredictable habitats should not be seen as the result of competitive exclusion from 'easier' habitats, but a requirement for long-term survival of these parthenogenetic animals."
The name "bdelloids" comes from the Greek and indicates their characteristic way of crawling in a leech-like manner, fixing alternately the terminal toes, extending, fixing the rostrum, and retracting the previously fixed end.
Although all the bdelloids do not always show the same behavior. For example a rotifer of the genus Adineta
moves normally by gliding over surfaces, using the cilia that cover the ventral side of their cephalic end.
Even the cephalic end, which gives the name to the class
<QUERY clarify>, does not have a homogeneous structure, and there exist at least 3 different structures, which allow division of the class into 3 Orders: Adinetida, Philodinida and Phylodinavida, and will be treated in detail when characterizing them.
As in the Monogononta (see Verolet's
Microscopies
article) the body is composed of 3 basic portions: the head, the trunk and the foot. The epidermis that covers it is a sincitial layer (that is to say, a layer of cytoplasm in which separated cells do not exist, although these are represented by large dispersed nuclei) and is marked by cross-sectional furrows that separate superficial rings (pseudosegments, since they do not divide the interior of the body), and that gives to the rotifer the capacity to be contracted in a "telescopic" way. This is possible thanks to the existence of a pliable layer of intra-cytoplasmic cuticle, called the "lorica".
In many Monogononta this lorica is hard and indeformable, with important specific characteristics. (See Verolet's
article.) But in the Bdelloidea it is almost always thin and folding, although in some species they can have characteristic thickening called "cuticular sculptures" (plates, furrows, spines, warts
etc).
The "corona", when it exists, can be enclosed inside the head, and this, and the foot at the other end, are contracted within the trunk, acquiring a compact and rounded form. This form (named "tun" in English) is the one that they adopt as a strategy of defense in any case of a sudden threat, or even as a previous step to drying. (See the above experiment by Christian Colin.)
4 - The immediate total retraction of a Philodina as a reaction to an abrupt contact, like a stroke over the coverslip.
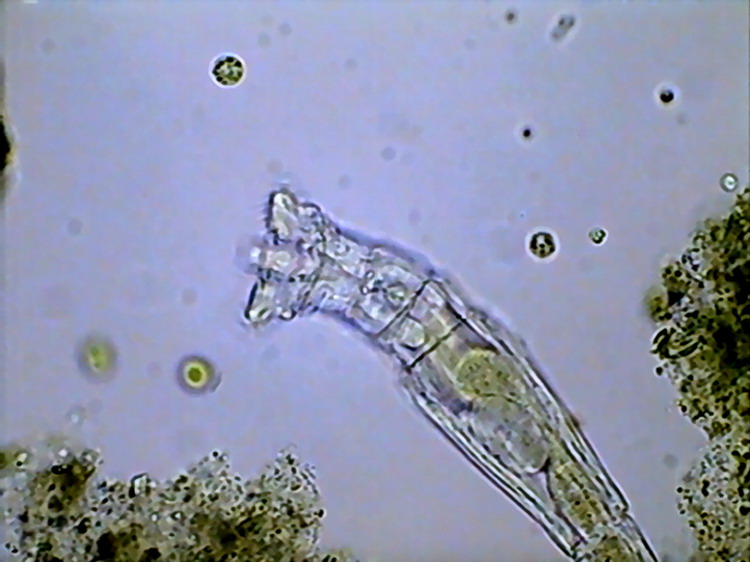
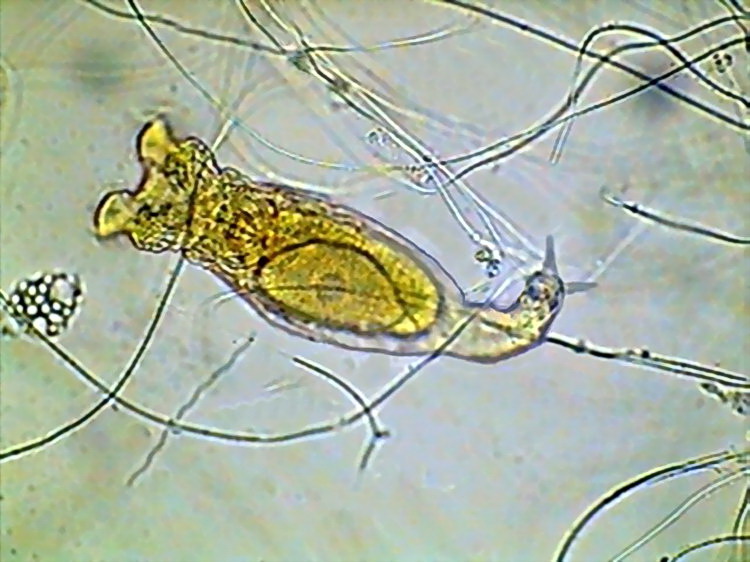
It is common that the head joint to the trunk has a narrowed portion called the "neck". Normally a sensitive antenna, thin, and more or less long, lodges dorsally in the "neck".
5 - Lateral view of a Philodina sp., showing the dorsal antenna. Below the antenna can be seen the red eyes. The pseudo-segmentation and the ridged trunk cuticle is also shown.
In addition, between the trochal
discs it can be seen dorsally the "rostrum", one generally robust structure provided at its end with cilia, sticky cells and sensitive lamellae. It is retractable in most of the species.
Some species have eyes, which lodge sometimes in the anterior end of the rostrum (Rotaria), and other times dorsally in the neck, over the brain (Philodina). In Adineta oculata they are described as
rostral eyes composed of a red spot surmounted by a concave lens.
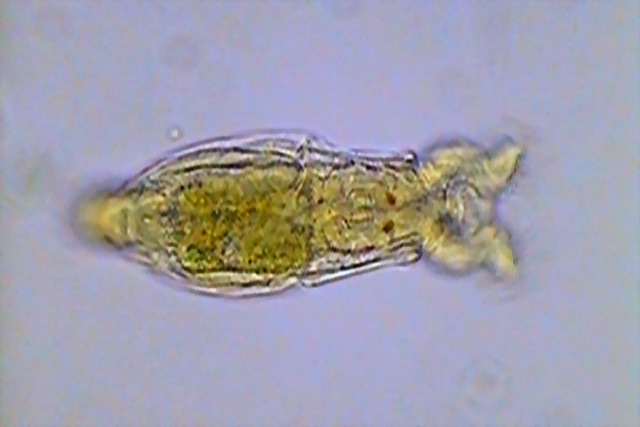
6 - Dorsal eyes over the brain in Mniobia. They are pigmented red.
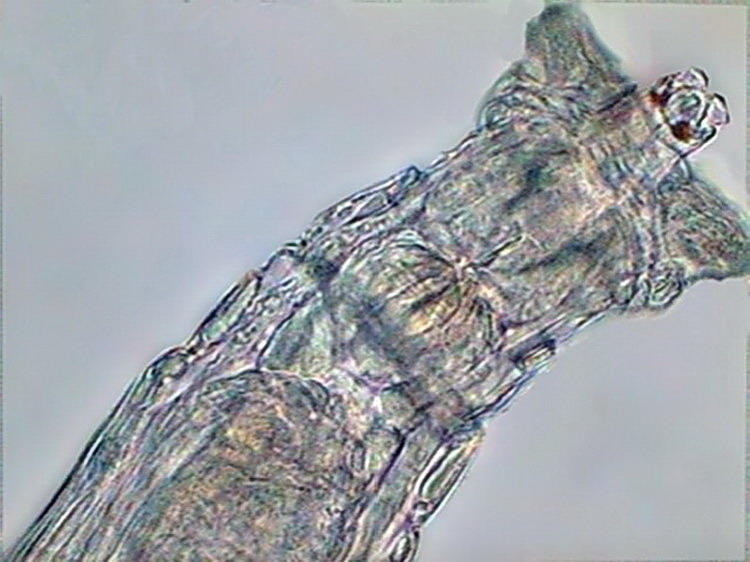
7 - Rotaria sp., dorsal view of the head, with dark eyes in the rostrum.
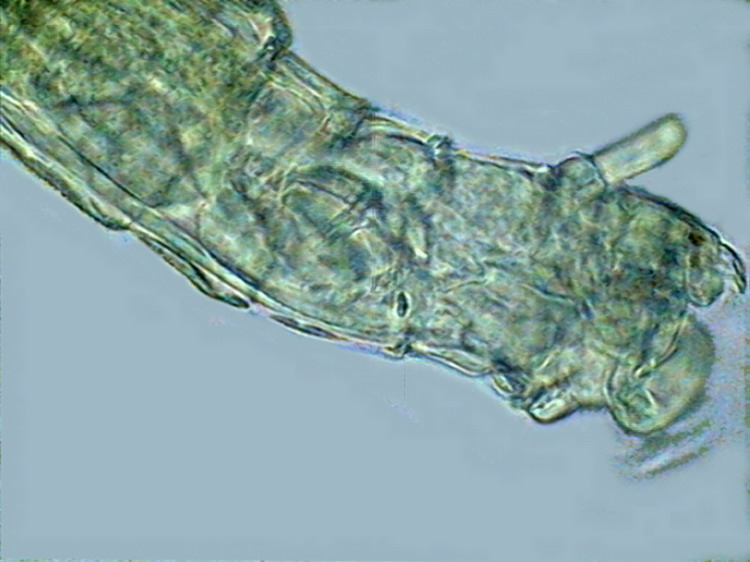
8 - Relation between the antenna and the rostrum. Trochal discs semi-retracted. One specimen with the "corona" totally retracted is seen in picture 27
(later article part).
The mouth opens centrally between the coronae (see fig 2) at the end of a buccal funnel, which could be followed by a buccal tube that communicates with a masticatory organ called the mastax.
The length of the buccal funnel and the buccal tube, and the depth of the mastax is a character that depends on the style of alimentation.

9 - The deep mastax of a Habrotrochidae.
You can see a mastax very near the mouth at fig. 30 (Henoceros),
later article part.
The mastax is a complex organ formed by strong muscles armed with several hard, cuticularized pieces, named "trophi". Its structure is characteristic of all the class and it really has little variety. The type of trophi of the Bdelloidea is called "ramate". The ramate trophi lacks the fulcrum, one unpaired piece characteristic of the remaining types of trophi of the phylum. (See Verolet's
article.). The trophi consist of 6 pieces, 2 unci (sing. uncus), 2 manubria (sing. manubrium) and 2 rami (sing. ramus). The plate-like unci are armed with teeth, differentiated into 3 groups. The anterior and posterior ones, are formed by thin teeth. There is a group (generally median) that has much more heavy teeth. According to the genus and the species,
these heavy teeth can number from 2 to 10.
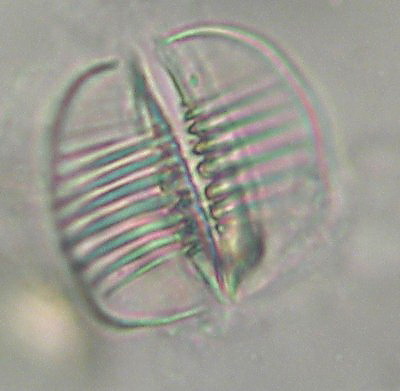
10 - Typical image of the trophi of the order Philodinida, courtesy of M. Verolet.
It can be compared with this image taken with an
electron microscope, and colored to differentiate the different pieces. In red the manubria, in blue the unci and in green the rami, underneath the indented edge of the unci.
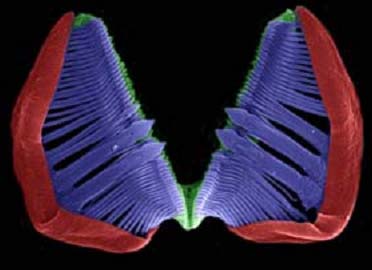
11 - SEM image of the ramate trophi electronically coloured, by G.Melone.
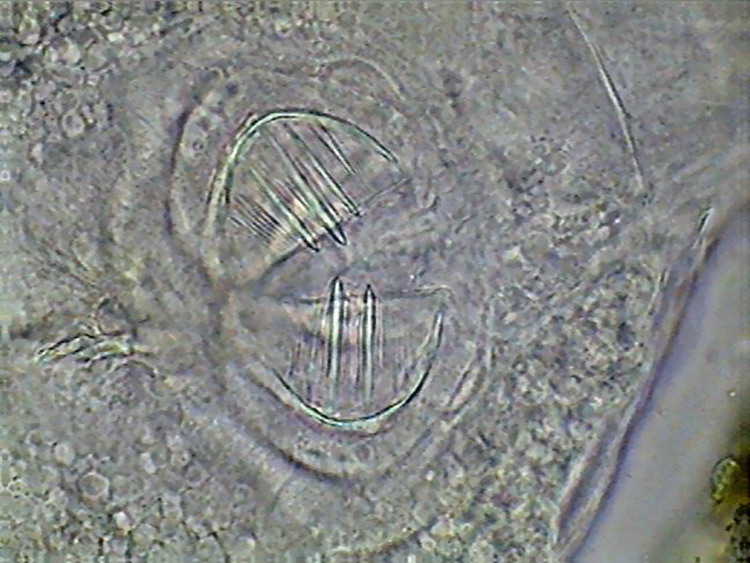
12
- Some atypical mastax of a Philodinavida
from Cancún. (5 images stacked with CombineZ 5.0.)
Moreover the trunk contains the remaining organs in its interior: brain, sub-cerebral and retro-cerebral organs, mastax, salivary glands, digestive and excretory systems, gonads, and muscles.
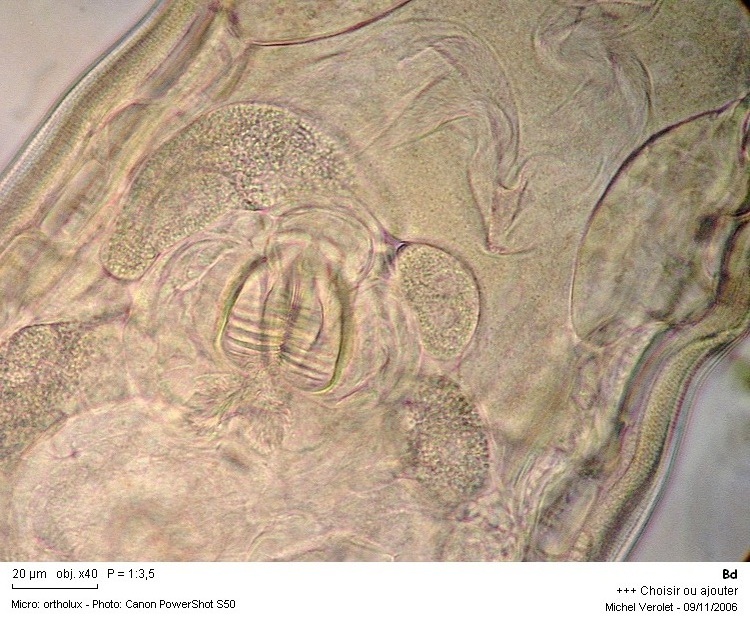
13a - Mastax, salivary, glands and intestine with heavily ciliated lumen in a bdelloid
rotifer. Courtesy of M. Verolet.
The mastax is followed by a normally very short esophagus, in most of the cases difficult to see, which opens into the sac-like, stretched, stomach. Normally the stomach is syncitial, with a ciliate cavity (mostly a tube) which is followed by a short, mostly bulbous, intestine (some times called "rectum"). But in the Habrotrochidae the stomach has no lumen at all and food is formed into little balls, and included in vacuoles that give the stomach a foamy appearance.

13b - The stomach of an Habrotrochidae.
Behind the rectum, or underneath, we can see the urinary bladder, which gathers the liquid expelled from the pseudocoel by two long protonephridia culminating near the neck by some flame bulbs. Bladder and rectum both finish in a common "cloaca" that opens to the outside dorsally in the base of the trunk.
The reproductive apparatus is represented by two germovitellaria, organs that produce eggs, and at the same time provide it with the vitelo (nutritive material) necessary for its development.
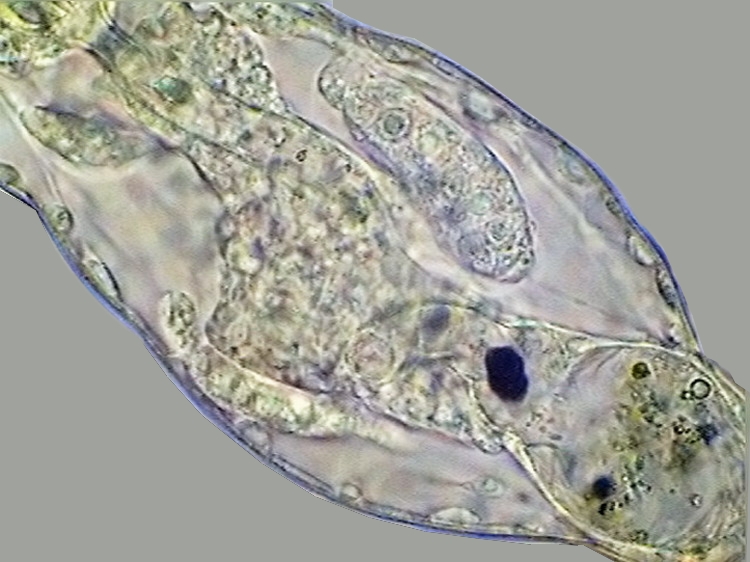
14 - Germovitellaria at both sides of the intestine, in the trunk of Adineta
sp.
Most of the Bdelloidea are oviparous.
15 - A fully formed egg, inside a bdelloid
rotifer.
But there exists some cases of viviparity. And sometimes the already formed embryos, even mobile ones, can be seen within the mother. Even if the development of the embryo is not advanced, viviparity can be denounced <confirmed?
QUERY> by the fact that the egg is seen as pluricellular.
16a - Segmented egg (embryo) inside Rotaria
sp. Note the absence of a shell and the irregular surface.
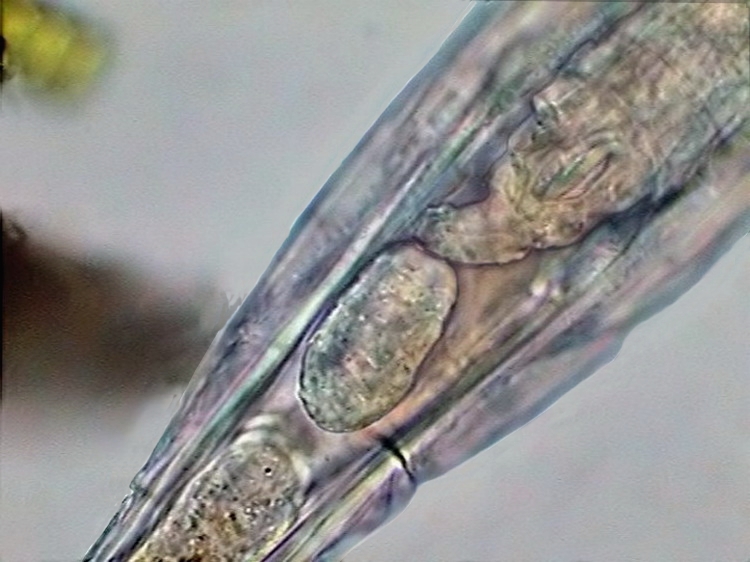
16b
- Embryo and another segmented egg in Rotaria sp.
The segments after the anus forms the retractable foot, at whose end 2 spurs can be normally seen, and behind these, a short structure lodge the toes, connected to glands, visible in the last portion of the foot, which produce sticky substances, allowing the rotifer to adhere to the substrate.
In a few cases (Bradiscella, Mniobia, and some epizoics) these toes are replaced by a sticky disc.
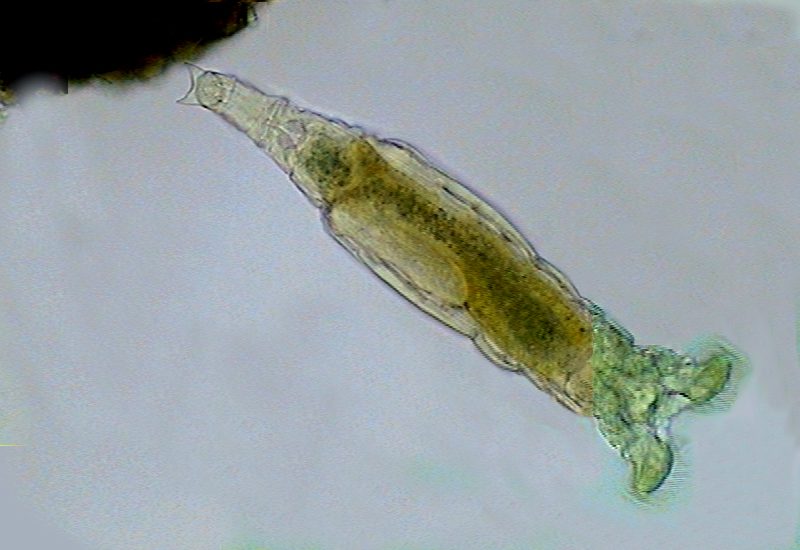
17a - Adhesive disc at the end of the foot in Mniobia.
17b - Spurs in the foot of Mniobia, note also the large egg.
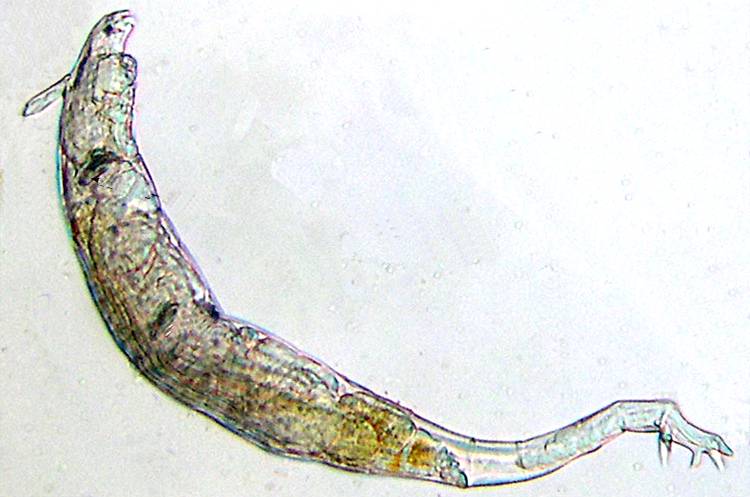
17c - Toes at the end of the foot of Rotaria (courtesy of Bertrand Parres).
I add here 3 tables with the list of the known genera, ordered under three different criteria. This can be redundant, but I think that it is an easy form to convey very interesting information.
LIST OF GENERA IN ALPHABETICAL ORDER
(in blue, epizoic genera)
Genus, author, publication date, approximate species numbers
|
Abrochtha |
Bryce |
1910 |
2 |
|
Adineta |
Hudson & Gosse |
1886 |
12 |
|
Anomopus |
Piovanelli |
1903 |
2 |
|
Bradyscela |
Bryce |
1910 |
2 |
|
Ceratotrocha |
Bryce |
1910 |
4 |
|
Didymodactylos |
Milne |
1916 |
1 |
|
Dissotrocha |
Bryce |
1910 |
7 |
|
Embata |
Bryce |
1910 |
5 |
|
Habrotrocha |
Bryce |
1910 |
100 |
|
Henoceros |
Milne |
1916 |
2 |
|
Macrotrachela |
Milne |
1886 |
100 |
|
Mniobia |
Bryce |
1910 |
50 |
|
Otostephanos |
Milne |
1916 |
9 |
|
Philodina |
Ehrenberg |
1830 |
40 |
|
Philodinavus |
Harring |
1913 |
1 |
|
Pleuretra |
Bryce |
1910 |
14 |
|
Rotaria |
Scopoli |
1777 |
24 |
|
Scepanotrocha |
Bryce |
1910 |
9 |
|
Zelinkiella |
Harring |
1913 |
1 |
A genus Callidina
still mentioned (especially in textbooks) is not valid now; it was very heterogenous and the described species have been distributed, between many of the now accepted genera.
2) Genera ranked by number of species
|
Habrotrocha |
Bryce |
1910 |
100 |
|
Macrotrachela |
Milne |
1886 |
100 |
|
Mniobia |
Bryce |
1910 |
50 |
|
Philodina |
Ehrenberg |
1830 |
40 |
|
Rotaria |
Scopoli |
1777 |
24 |
|
Pleuretra |
Bryce |
1910 |
14 |
|
Adineta |
Hudson & Gosse |
1886 |
12 |
|
Otostephanos |
Milne |
1916 |
9 |
|
Scepanotrocha |
Bryce |
1910 |
9 |
|
Dissotrocha |
Bryce |
1910 |
7 |
|
Embata |
Bryce |
1910 |
5 |
|
Ceratotrocha |
Bryce |
1910 |
4 |
|
Abrochtha |
Bryce |
1910 |
2 |
|
Anomopus |
Piovanelli |
1903 |
2 |
|
Bradyscela |
Bryce |
1910 |
2 |
|
Henoceros |
Milne |
1916 |
2 |
|
Didymodactylos |
Milne |
1916 |
1 |
|
Philodinavus |
Harring |
1913 |
2 |
|
Zelinkiella |
Harring |
1913 |
1 |
3) Genera ordered by the publication year
|
Rotaria |
Scopoli |
1777 |
24 |
|
Philodina |
Ehrenberg |
1830 |
40 |
|
Macrotrachela |
Milne |
1886 |
100 |
|
Adineta |
Hudson & Gosse |
1886 |
12 |
|
Anomopus |
Piovanelli |
1903 |
2 |
|
Habrotrocha |
Bryce |
1910 |
100 |
|
Mniobia |
Bryce |
1910 |
50 |
|
Pleuretra |
Bryce |
1910 |
14 |
|
Scepanotrocha |
Bryce |
1910 |
9 |
|
Dissotrocha |
Bryce |
1910 |
7 |
|
Embata |
Bryce |
1910 |
5 |
|
Ceratotrocha |
Bryce |
1910 |
4 |
|
Abrochtha |
Bryce |
1910 |
2 |
|
Bradyscela |
Bryce |
1910 |
2 |
|
Philodinavus |
Harring |
1913 |
1 |
|
Zelinkiella |
Harring |
1913 |
1 |
|
Otostephanos |
Milne |
1916 |
9 |
|
Henoceros |
Milne |
1916 |
2 |
|
Didymodactylos |
Milne |
1916 |
1 |
No new genera has been described in the last 92 years
|