|
Note on 'diatom dotting' at low magnifications. Resolving Stauroneis phoenicenteron. by David Walker, UK |
Resolving the fine detail of diatom frustules remains a popular pursuit of microscopy enthusiasts including myself. It tends to be more associated with the use of high NA dry and immersed objectives to resolve the more demanding species with fine striae and/or pore detail.
As discussed and illustrated in a Sept 2008 Micscape article studying Pleurosigma angulatum, resolving less demanding diatoms at lower objective mags can be fun and instructive. They are useful subjects to check the quality of the entire optical train as well as demonstrating some basic principles of microscopy such as condenser quality / iris setting, light wavelength effect on resolution and the quality of a photomicrography setup.
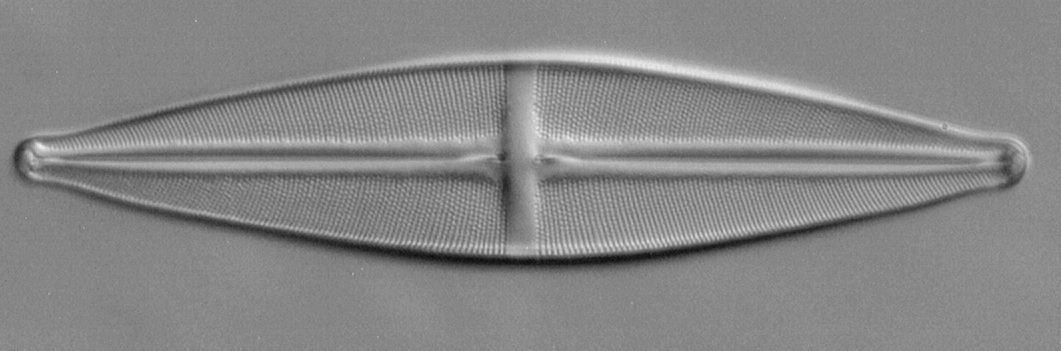
Another species of diatom frequently found on test slides is Stauroneis phoenicenteron Nitsch (Ehrenberg) and included on test slides by master preparers i.e. Klaus Kemp's 'Test Plate 8 Forms' and Stefano Barone's (of Diatom Lab) 'Diatom Test Slide version 2.0'. The species is typically studied with a 40X objective where the pores should be readily resolved in brightfield but a 400 nm filter crisps them up for more accurate measurements in an A4 printout as shown below using the Diatom Lab test slide.
Measuring the striae and pore separations first at a higher mag. Before studying at lower powers, measuring the striae and pore separation on own example is worthwhile as there can be some variability (Diatom Lab on their valuable support page for their test slide, link above, notes 12-15 'longitudinal striae' in 10 µm). Modern microscope camera software can measure on-screen images after calibration but I prefer to print an A4 image with an accompanying image of a micrometer scale to calibrate the page. This allows a length of pores/striae to be measured for a good average to be taken. My Diatom Lab example shown below had 15 striae to 10 µm, 0.714 µm apart midline to midline. Pores were 0.63 µm between pore centres. Using a typical equation (see Footnote 1) this suggests an NA of 0.53 is required to resolve the pores.

Zeiss Photomicroscope III, ach-apl condenser, Neofluar 40/0.75, Optovar 2X, 400 nm filter (with 100W halogen), slight oblique. Opticstar PL-130M 1.3 Mpixel astro' monochrome camera.
Diatom Lab Test Slide version 2.0. Proprietary 'Diatom Cubed' mountant with frustules attached to underneath of 'high optical quality' coverglass.
Some levels adjustment, crop of master no sharpening in Photoshop Elements 17.0.
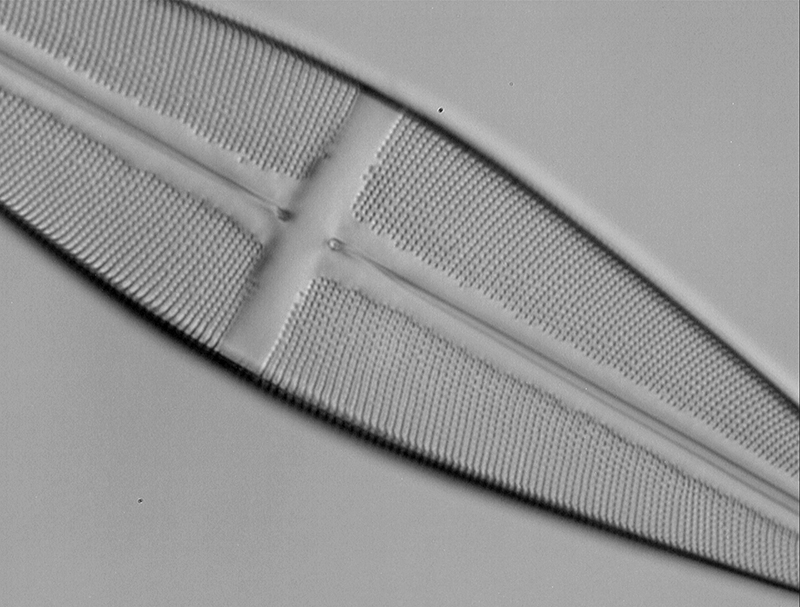
Resolving with a 16/0.4 objective. Any 40X objective with NA typically of 0.65 should readily resolve striae and pores. The Zeiss Neofluar 16X with NA0.4 does not in brightfield. But using a 400 nm interference filter on the field lens using the near UV present in an unblocked 100W halogen filter (see last month's Micscape) and monochrome camera should in principle increase its NA to 0.55 (decreasing wavelength from the 500 nm used in the equation to 400 nm). The image below was taken with the Neofluar 16/0.4 with 400 nm filter. The Optovar was set to 2X as empty mag is sometimes useful as in this case so that it fills a greater number of sensor pixels. Striae and pores are clearly fully resolved.
Stauroneis phoenicenteron. Zeiss 16/0.40 Neofluar, 2X Optovar, 400 nm filter, slight oblique.
Camera, image processing as above. Diatom Lab Test Slide version 2.0.
Resolving with a 10/0.45 objective. Modern 10X planapos typically have NAs of ca. 0.45, such as the Nikon CFI objective used on my Eclipse 800. Again the 400 nm filter should allow resolution of pores but I have struggled to capture an image because the 1.3 Mpixel camera has insufficient resolution. The Eclipse also has no Optovar and a 2X C-mount relay lens was not enough to fill the camera field beyond third of sensor width. The photo port of the Eclipse also does not respond to increased mechanical tube length to increase the area covered on the sensor. The screen capture below when image zoomed to 200% shows the problem. At 400 nm of course cannot rely on a visual assessment to see if resolved and likely bordering on visual acuity. But will explore further!
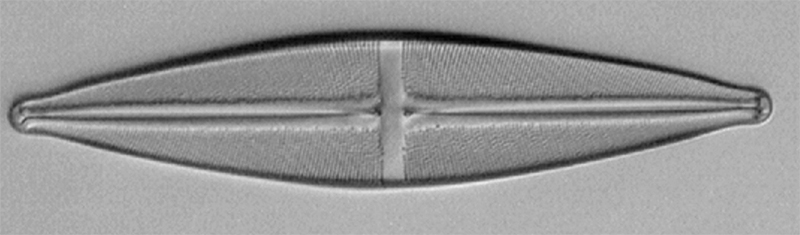
Stauroneis phoenicenteron. Nikon Eclipse 800, ach-apl condenser, slight oblique, Nikon CFI 10/0.45 planapo, 2X relay lens on photo port.
Screen capture of 200% screen mag.
Footnotes:
1)
The textbooks discuss various forms of the equation linking resolution R (nm
or µm)
to wavelength l (nm
or µm)
and NA of objective.
The equation R = 0.61 x l /
NA was used.
Acknowledgement: The author used the invaluable 'Test Slide version 2.0' (Diatom Cubed mountant) supplied by Stefano Barone of Diatom Lab.
Comments to the author David Walker are welcomed.
Published in the September 2021 edition of Micscape.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK web site at Microscopy-UK
© Onview.net Ltd, Microscopy-UK, and all contributors 1995
onwards. All rights reserved.
Main site is
at www.microscopy-uk.org.uk
with full mirror
at www.microscopy-uk.net
.