 2) An admittedly self-indulgent sharing of my school level studies.
2) An admittedly self-indulgent sharing of my school level studies.|
Reflections on studying Spirogyra - a classic school biology subject and plenty of interest for the hobbyist.
by David Walker, UK
|
One of the more distinctive filamentous algae is Spirogyra with its spirally arranged chloroplasts. For the microscopy hobbyist it offers plenty of interest and Micscape contributors have shared a selection of articles (see Related Micscape Articles section). This article concentrates on the aspects below from my own recent studies:
1) Spirogyra under a commercial Van Leeuwenhoek replica microscope. My own interest in this algae was piqued when helping Wim van Egmond share his February 2016 Micscape article The Riddle of the 'green streaks'. Antoni van Leeuwenhoek: In search of the first microorganism he described. In this article he reassesses whether Leeuwenhoek did first describe Spirogyra in his letter to the Royal Society dated Sept. 7th 1674. Wim, in collaboration with a phycologist colleague Frans Kouwets, presents persuasive arguments that the later attribution of Spirogyra was not the most likely candidateanother organism matches the features in Leeuwenhoek's description much more closely. We agreed that it would be a useful complement to share images of Spirogyra taken through a replica Van Leeuwenhoek microscope.
 2) An admittedly self-indulgent sharing of my school level studies.
2) An admittedly self-indulgent sharing of my school level studies.
3) The usefulness of Spirogyra for exploring different lighting techniques including autofluorescence with simple filter additions to a typical transmitted compound microscope with darkfield facilities.
4) A good resource for attempting identification to species in Britain.
5) Some typical commercial prepared slides, including the set offered by the late Eric Marson of Northern Biological Supplies (NBS) showing conjugation.
Sourcing the Spirogyra
My back garden pond rarely has filamentous algae and not to date Spirogyra, but I recalled sampling a local water trough some years ago where it did occur. In early February 2016 on a return visit there was a substantial algal growth and was pleased to find that this was a near mono-culture of Spirogyra and as a bonus it had been conjugating.
Right, June 2004 photograph. Water troughs hewn out of the local millstone grit (a coarse-grained sandstone) are an interesting upland habitat in the north of England where I live (see my June 2004 Micscape article). Some, like this spring fed trough, could be regarded as a slow moving river with its continuous water flow for most of the year. They also offer varied habitats in a small area, e.g. splash zones with bryophytes, damp mud, open water, rocky substrates etc.
Spirogyra viewed under a Leeuwenhoek replica microscope
 As is well known, Leeuwenhoek made his own single lens microscopes for his studies. Single lenses were superior to compound microscopes of the time and remained so until the development of achromatic microscope objectives in the early 19th century. I have two commercial replicas, the one shown below and the second sold by the Museum Boerhaave. Both have similar magnifications but prefer the former for use as it's somewhat larger and easier to handle.
As is well known, Leeuwenhoek made his own single lens microscopes for his studies. Single lenses were superior to compound microscopes of the time and remained so until the development of achromatic microscope objectives in the early 19th century. I have two commercial replicas, the one shown below and the second sold by the Museum Boerhaave. Both have similar magnifications but prefer the former for use as it's somewhat larger and easier to handle.
Left. A modern brass replica made by Chris Kirby of Christopher Allen Replicas (UK) with simply engineered parts and aged to look authentic in a style typical of Leeuwenhoek's designs.
It is shown from the subject's side and has to be held close to the eye from the other side. The single lens is fixed between two riveted brass plates with dimpled apertures. The three screws allow focussing and subject orientation. The subject is mounted on the pin.
This replica is stated to have a ca. 100X magnification (at 250 mm) and was confirmed by my own measurements. The focal length is ca. 2.5 mm and subject field of view presented to the eye is ca. 0.8 mm.
 Right. The replica mounted on a Sony NEX 5N digital camera body. There are no optical components other than the replica lens. The assembly was mounted on a tripod and pointed at either a curtained window (to control light aperture) or an indoors lamp. Note that the adaptor was one to hand, but any lensless mechanical extension suitable for the camera mount can be used e.g. one or more extension tubes. The amount of extension will change the field captured which could be chosen as the full circular field of the lens or a greater extension to crop the field to suit.
Right. The replica mounted on a Sony NEX 5N digital camera body. There are no optical components other than the replica lens. The assembly was mounted on a tripod and pointed at either a curtained window (to control light aperture) or an indoors lamp. Note that the adaptor was one to hand, but any lensless mechanical extension suitable for the camera mount can be used e.g. one or more extension tubes. The amount of extension will change the field captured which could be chosen as the full circular field of the lens or a greater extension to crop the field to suit.
The lens to sensor distance was 63 mm i.e. much less than the traditional 250 mm projection distance for formal studies. This projection distance was more practical for the setup and image just filled the APS sensor. A magnification more typical of what is seen by the eye is restored in either a screen or printed image.
In letters earlier than Sept. 7th 1674, Leeuwenhoek described his use of fine capillary tubes for studying liquids such as blood or milk and may have used such tubes for his aquatic samples from Berkelse Lake. As Wim notes in his article, if he was reporting Spirogyra using such tubes it must have been a challenge persuading the long filaments to enter a narrow tube compared to the more likely candidate which Wim suggests.
Leeuwenhoek is known to have also used mica plates or thin blown glass to mount aqueous subjects attached to the pin (1) and I adopted a similar method as shown using coverslip pieces but with a more suitable support. Hans Loncke shows modified pin designs for his work with the splendid Leeuwenhoek replica that he built (2). Perhaps Leeuwenhoek also made alternative supports to the pin for work with flat plates.
Spirogyra viewed under a Leeuwenhoek replica microscope. Optical mag 100X. Typical filament diameters 36 µm.
A darkened room with a vertical narrow light source was used to mimic Leeuwenhoek's suggestion for best use of his microscopes i.e. with a restricted aperture (3,4).
|
|
The Christopher Allen replica uses a hand ground 'convex' glass lens by the maker Chris Kirby (5). Leeuwenhoek was known to have made and used ground lenses or blown aspheric lenses (6). The lens shows the cellular structure of the filament clearly and the spiral chloroplasts and pyrenoids. Residual aberrations aside, the image differs not that much from a modern achromatic objective on a compound microscope, see later section. |
|
|
The same filament as above except using darkfield. Leeuwenhoek could not have failed to create and value the use of darkfield illumination with appropriate subjects. When setting up the microscope with a narrow light source, until the lens is fully aligned a slightly off-axis view readily creates this form of lighting. For a discussion of Leeuwenhoek's likely use of darkfield see Snyder (4) and Dobell (7). |
|
|
The upper filament may be in the early stages of conjugation (or a different species!).
|
|
|
A more general view of multiple filaments with ca. 50% of the full visual field is shown. The visual field of view of this 100X lens is ca. 0.8 mm which is comparable to that of a 16X modern compound microscope objective with 10X eyepiece (field. no. 18) and Optovar set at 1.25X on my Zeiss Photomicroscope III. |
School studies of Spirogyra in the early 70s
My first encounter with Spirogyra was in the early 70s when studying for the GCE 'O' level exam in biology at high school. At that time, the course included studies of typical examples of various groups. Amoeba was the single-celled animal, spirogyra the algae and advancing to more
complex organisms like the hydra. A browse through some equivalent exam level textbooks using Amazon UK's 'Look Inside' feature suggests that this isn't now a typical modern approach in the UK.
They say that you never forget the schoolteachers who were of most influence and that is certainly the case for me. Mr Tann, the biology teacher at King Edmund Comprehensive, Rochford, Essex in the early 70s presented biology with an infectious enthusiasm and covered many aspects that still engage me as a hobbyist today. Practical microscopy work used the LOMO Biolam, a model that I later bought and have used as a hobbyist for over 35 years. Being a bit of a hoarder, I still have my school notes when aged 15 and the drawings are shown below.
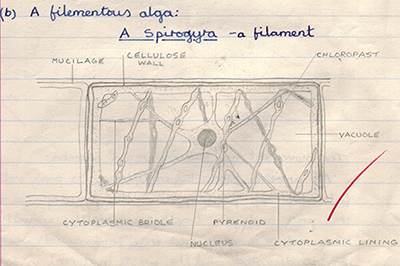
Above. Course work that likely required a redrawing of the diagram of an idealised cell from the accompanying textbook.
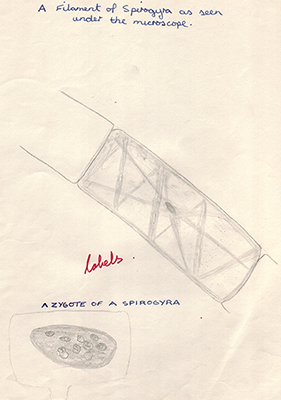
Left above. Own view of a specimen as seen under the LOMO Biolam microscope which were widely used in practicals.
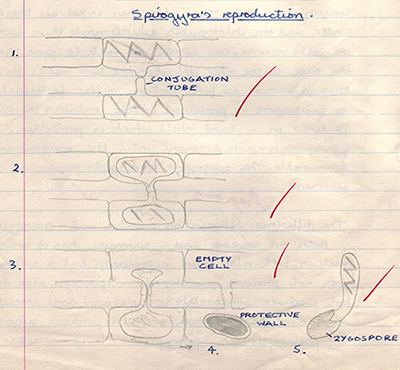
Right above. Spirogyra was a popular example of an algae, partly because of its distinctive forms of sexual reproduction. The scalariform of reproduction is shown.
Exploring Spirogyra using different lighting techniques including transmitted autofluorescence using a darkfield stop
The ease of preparing temporary fresh mounts of algae such as Spirogyra and the variety of forms it offers if undergoing conjugation, make it an interesting subject to study. Chlorophyll is noted for its relatively bright autofluorescence (cf. weakly emitting fluorochromes) and it is possible to use a normal transmitted compound microscope using a darkfield stop and a couple of filters to explore autofluorescenceno special epi-fluorescent microscope with intense lamp is required. Although a digital camera with good long exposure capabilities is ideal to record and study the results.
The images below used a Zeiss Photomicroscope III with Canon 600D DSLR body with Zeiss 10x Kpl eyepiece on short collar for projection. The 'D' setting on a water immersed achromatic-aplanatic condenser was used for both the darkfield and the autofluorescence studies.
|
The next three images show the same view of fresh Spirogyra in a temporary water mount under different lighting conditions. Phase with Zeiss 10/0.22 achromatic phase objective. One filament shows the completion of scalariform conjugation where the contents of one filament (designated the 'male') are transferred to a second (the 'female') to form a zygote. |
|
| Same 10/0.22 objective with darkfield by using the larger Ph3 annulus (condenser water immersed). |
|
| Zeiss 10/0.32 planapo objective with the Ph2 disc to create circular oblique illumination or COL (condenser water immersed). |
|
| Zeiss Neofluar 25/0.65 objective with DIC and the type II prism. The shallow plane of focus provides a clearer view of the spiral chloroplasts and pyrenoids compared with the same view using phase below. |
|
|
As above but using phase which gives a rather muddled view cf DIC. Rather intriguingly, I was struggling to see either the nucleus or the cytoplasmic bridle, despite all the firepower the PMIII techniques offered. I had clearly drawn these features at school as shown earlier using brightfield on the LOMO. Whether they were indeed very clear for that species or it was wishful thinking knowing what the ideal cell in the textbook looked like, I'm not certain! |
|
|
Autofluorescence of the chlorophyll containing chloroplasts shows the spiral structure well. Zeiss 10/0.32 objective with darkfield using the water immersed 'D' setting on the condenser. The filters best used for chlorophyll autofluorescence from past experience were intentionally 'leaky' i.e. some blue excitation light was allowed to pass the barrier filter as it defines non-fluorescing components e.g. the cell walls in pale blue darkfield. Small e.g. 18 mm filters as used in the Zeiss III RS head are fine. Excitation, 1 Schott BG12 filter (two would be usual for the deep blue excitation set in the III RS epi head). CM500S filter (or a BG38) to remove residual red from the light. Both filters sit on the field lens plate. Barrier filter. Barrier '478 nm' filter from the III RS epi head. The barrier '500 nm' should be used for the Zeiss deep blue set but the 478 nm lets some blue pass. This filter sits in the PMIII filter holder supported on a card collar. |
|
|
This and the following image are of the same view to compare phase and autofluorescence. Two parallel filaments have completed the scalariform form of sexual reproduction. The zygotes are shown and the empty 'male' filament cells. Zeiss Neofluar 16/0.4 objective. |
|
|
Same subject and field of view as above but autofluorescence. The chlorophyll rich zygotes show well in addition to the chloroplasts in the lower filament. Exposure 30 secs ISO 800. |
|
 An attempt at identifying to species
An attempt at identifying to species
Identification to species usually requires the reproductive forms to be present. As this was the case with the sample above collected from the water trough, I had a stab at an ID. I'd treated myself some years ago to the splendid The Freshwater Algal Flora of the British Isles edited by John, Whitton and Brook pub. 2002 (shown right with dish of Spirogyra). This was a more affordable two figure sum at the time but the second edition pub. 2011 is now typically £140. The flora includes a key, supported with many illustrations, to the 50 or so species of the 400 in the Spirogyra genus which they note have been reported in the British Isles.
For the dominant species present, the typical measurements were: filament diameter 36 µm, cell length 68 - 130 µm, ellisoidal zygotes 58 µm x 30 µm Chloroplasts 1 per cell? If I've keyed out correctly (having never used the key before) it is Spirogyra varians (Hassall) Kützing 1849. Noted as 'probably cosmopolitan'. Mountain streams are included as a habitat which the slow flow through the upland water trough it was collected from could be regarded as. Although a feature noted on the zygotes for this species is 'mostly with a distinct suture line' which I could not see.
Some commercially prepared slides
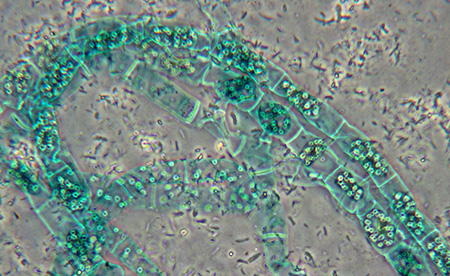
The late and sadly missed Eric Marson of Northern Biological Supplies (NBS) offered a splendid set of fluid mount spirogyra slides. They included examples of both the scalariform and lateral forms of sexual reproduction. The former is the commoner 'ladder' form where two filaments come side by side, the lateral form is where adjacent cells in the same filament undergo reproduction. Sadly, only one of the fluid mounts is still in good condition, the others have dried out and are now unusable. I believe that one or more of these samples also formed the basis of Eric's paper which he published in the Quekett Journal (8) on his meticulous observations on aspects of selected Spirogyra species and their reproduction.
Many of the good value large slide sets contain examples of spirogyra. My brother Ian and I have an unbranded 100 slide set with two examples as shown. Unfortunately, this may be an example of false economy rather than selecting slides of particular interest from a well established preparer. The quality of the mounts are variable and the mount has crystallised in many slides, making any photography using contrast enhancement unsuited as shown. Note also the curious labelling 'Spriogyra Conjugation' and 'Spirogation'.
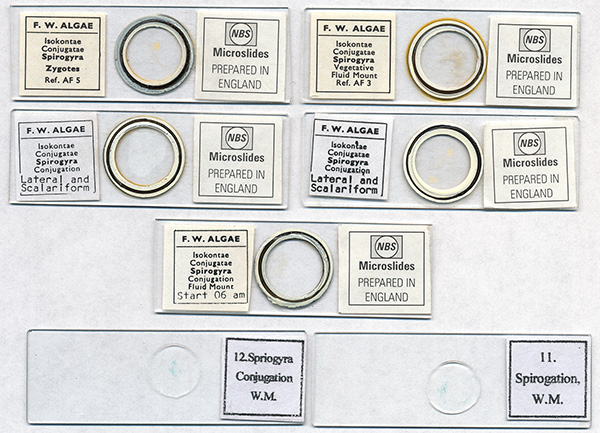
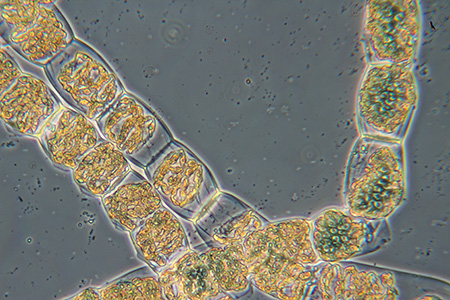
Left, filaments from the 'Spirogyra, Conjugation, Fluid Mount, Start 06 am' slide by NBS above. The slide remaining fluid from the set.
Right, filament from the unbranded slide 12 shown above. The mount has crystallised giving muddled views if contrast enhancement is used. The filaments looks as if they have been stained.
Both slides using a Zeiss 10/0.22 achromatic phase objective in phase.
The author David Walker welcomes any comments / corrections.
References
1. M. Folkes, 'Some account of Mr. Leeuwenhoek's curious Microscopes, lately
presented to the Royal Society', Philosophical Transactions, 1724, XXXII, 446. As
quoted by Dobell ref. 7, p. 316. (Link is to open access paper.)
2. e.g. Hans Loncke in his Micscape July 2007 article 'Making an Antoni van Leeuwenhoek
microscope replica'.
3. Brian J. Ford, 'The Leeuwenhoek Legacy', 1991, Biopress, London, p.73 side note who cites ref. 3b.
3b. Antoni van Leeuwenhoek in his letter dated June 1st 1674 to H. Oldenburg Secretary of the Royal Society, The Collected Letters of Antoni van Leeuwenhoek = Alle de Brieven van Antoni van Leeuwenhoek, 1939, vol. I, letter no. 8 [4], p.115. (Link is to open access letter.)
4. Laura J. Snyder, 'Eye of the Beholder. Johannes Vermeer, Antoni van Leeuwenhoek, and the Reinvention of Seeing', pub. Head of Zeuss Ltd., 2015, p.294-5 who cites ref. 4b.
4b. Barnett Cohen, 'On Leeuwenhoek's Method of Seeing Bacteria', J. of Bacteriology, 1937, 34(3), 343-346. (Link is to open access paper.)
5. Phil Greaves, 'Replica Leeuwenhoek microscopes', on the Quekett Microscopical Club website accessed February 2016.
6. J. van Zuylen, 'The Microscopes of Antoni van Leeuwenhoek', J. of Microscopy, 1981, 121/3, pp. 309-328. (Reprinted in 'Antoni van Leeuwenhoek, 1632-1723', Eds. L. C. Palm and H.A.M. Snelders, Rodopi, Amsterdam, 1982, pp.29-55.)
7. C. Dobell, 'Antony van Leeuwenhoek and his "Little Animals"', first published 1932. Dover Edition 1960, p.331. (Link is to a free copy on www.archive.org.)
8. J. E. Marson, 'Observations on Sexual Reproduction in species of Spirogyra', Microscopy (The Journal of the Quekett Microscopical Club now the Quekett Journal of Microscopy), 1992, vol. 36 (part 9), 712-717, 720.
Acknowledgement
Thank you to the Digitale Bibliotheek voor de Nederlandse Letteren (DBNL) website for both hosting and making freely accessible the first fifteen volumes of the 'Collected Letters of Antoni van Leeuwenhoek'.
Related Micscape articles
'Spirogyra' by Jan Parmentier, Micscape January 1999.
'Conjugation in Spirogyra', by Wim van Egmond, Micscape 1998. Part of Wim's The Smallest Page on the Web suite.
'Forays into fluorescence. Simple transmitted blue light autofluorescence of mosses and algae imaged with a digital SLR.' by David Walker, Micscape March 2009.
Revision history.
First published February 13th 2016.
Various amendments, corrections and additional references added up to and including February 20th 2016.
Published in the February 2016 edition of Micscape.
Please report any Web problems or offer general comments to the Micscape Editor .
Micscape is the on-line monthly magazine of the Microscopy UK web site at Microscopy-UK
© Onview.net
Ltd, Microscopy-UK, and all contributors 1995 onwards. All rights
reserved.
Main site is at www.microscopy-uk.org.uk
with full mirror at
www.microscopy-uk.net
.