As I state in the
paragraph on methods of
observation and documentation, the simple photographs taken by
most microscopists do
not
allow a precise species determination. Names applied to the specimens
shown
here are
compatible with the key. But the absence of any complementary data
only allows
"probable" identifications. I hope that in the future any
microscopist having a Stentor
under their objectives (including me, of
course, if
I have the chance to find them anew) compiles all the relevant
information which they will need to do a positive identification.
INTRODUCTION
One genus
which readily draws the attention of microscopists who observe
the freshwater protists, is Stentor,
whose first species was named 231
years
ago. Generally of large size, sometimes numerous, and very frequent,
the stentor
is easy to study even with low powers. The desire to give a
denomination (a
specific name) to the studied specimen conflicts with the shortage of
descriptions in the reference books.
My first
intention was to produce a small key of the more common species
of the genus. But, aside from the difficulty in deciding which species
to
exclude, the study of the subject made me more interested in a broader
key, to encompass not
only the Stentor
genus, but also the family which includes it and two
other closely related families. This article is the result of all these
inquiries.
The heavily annotated key
that I add at the end, tries to offer microscopists the best
and
fastest
taxonomic determination of their specimens. One cannot however regard
this method
as infallible. A few characters from one key, even if they are
important, are
not enough to replace a complete description of the species. The big
task
that faces the taxonomist (professional or not) who is interested for
the first time in the stentors
is clearly illustrated by the number of publications on the genus,
which,
according to Foissner and
Wölfl 1994 exceeds 1300.
Individuals
of each species can present great
variations, which can require for their definition a somewhat
deeper
study, with advanced techniques, like silver impregnation for
example. The
specialists have not even been able to completely agree on the
characters that are
used to define and to differentiate the Stentor
species.
There exists however
a
series of characters which can be observed
without ambiguity with average powers and which make it possible to
attempt
the primary identification of even live specimens. These characters
are, in order of ease
of
observation:
2 - the shape and size of the nucleus,
3 - the form of the apical sector or peristomial bottom, and the presence or not of one "buccal pouch",
4 - the presence or not of pigmented granules between the longitudinal lines of cilia
5 - obviously the color of the pigment if it is present
6 - the existence or not of a total or partial gelatinous tube (lorica) in which the individual lives
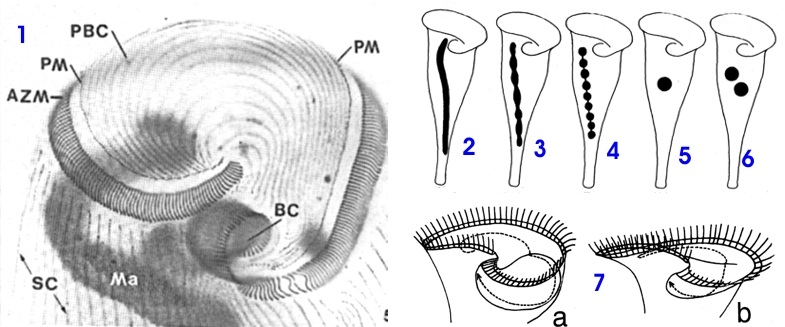 |
| Fig. 2 - description of the anterior end of one Stentor roeselii (silver impregnation): AZM, Adoral Zone of Membranelles. BC, oral cavity. Ma, Macronucleus. PBC, Ciliary lines of the "peristomial bottom", PM, parorale membrane. SC, ciliary somatic lines. Figs. 2-6, different nuclear forms: 2 vermiform, 3 nodular, 4 moniliform (in chain), 5 only one ball, 6 more than one ball. Fig. 7a- peristomial bottom with a "buccal pouch", 7b shows a peristomial bottom without "buccal pouch". (One of the lines of cilia of the peristomial bottom is drawn for showing better the position and form of the pouch. Figs 1-6 modified from Foissner and Wölfl 1994, Fig. 7 modified from Kumazawa 2002. |
 |
 |
 |
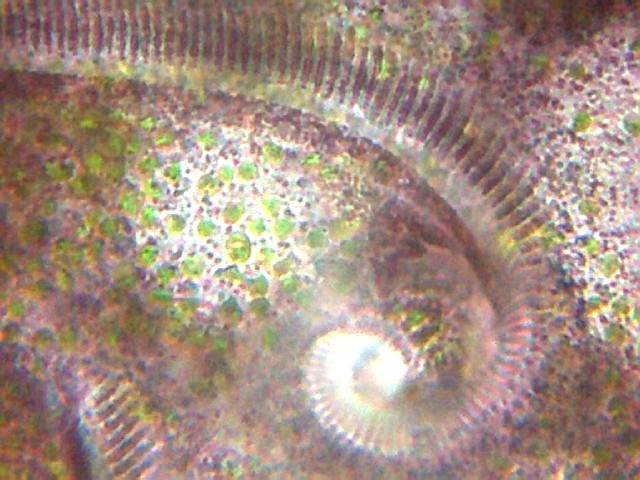
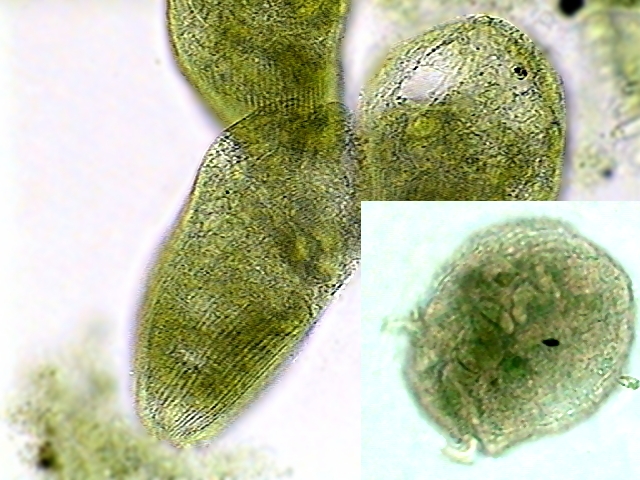
| Fig.
3 Stentor amethystinum - AZM
sinking to the mouth. In this and the following pictures we can see the
ectoplasm with its pigment granules and the zoochlorelles. |
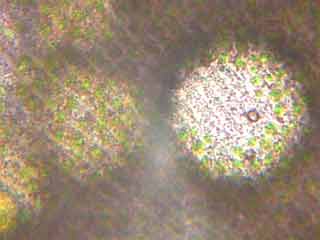
Fig. 4 Stentor amethystinum - disposition of the ciliary lines (clear lines) and the bands of pigmented granules. The background is strewn with zoochlorelles. | Fig. 5 Another species, showing the cytoplasm deprived of zoochlorelles, but with food vacuoles full with chlorelles. See the moniliform nucleus. |
| above three images are from Christian Colin | ||
 |
 |
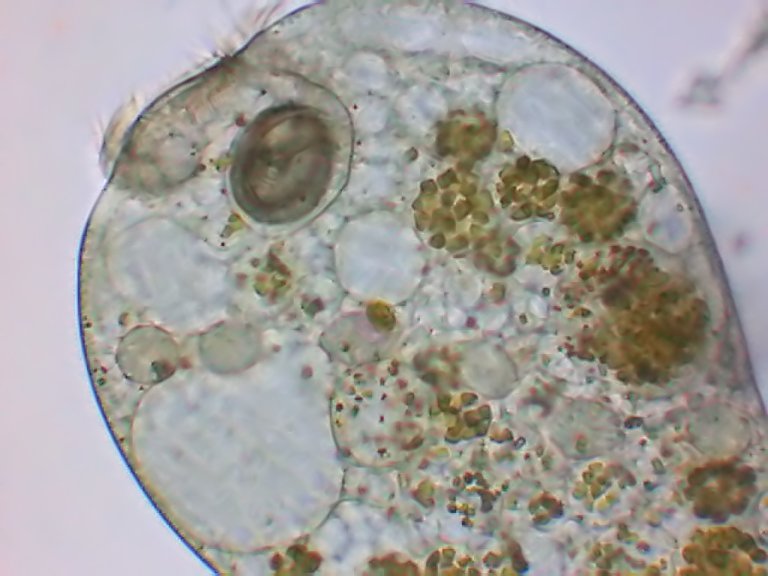
| Fig. 6 - prob. St. Muelleri from Cancún.
We can see the lines of ciliary insertion. Fixed with hot AFA, stained
with Fast Green, mounted in NPM. In the right insert the moniliform nucleus
of
ellipsoidal beds is shown. |
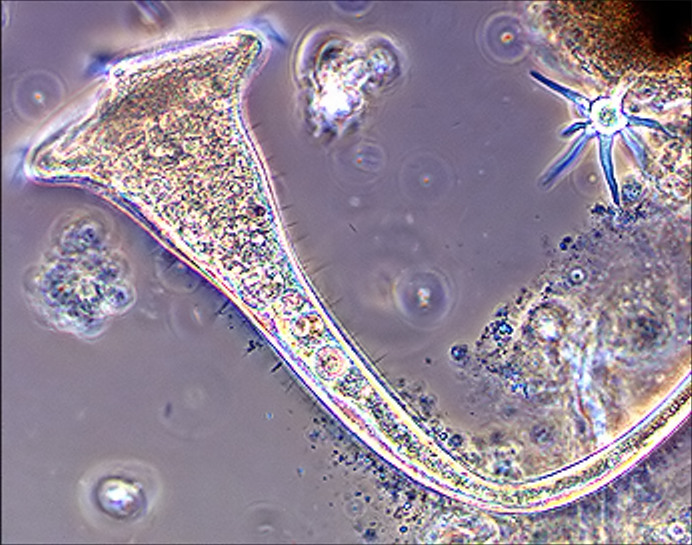
Fig
7 - Stentor
picture in phase contrast. Although the inner details are not
very visible, cilia differentiate in short somatic cilia and stereocilia
or rigid cilia, more dispersed than the somatic ones. |
|
Fig. 7 reproduced by the kindness of Rick Gillis, PH.D, from his site HTTP://bioweb.uwlax.edu/zoolab/Table_of Contents/Lab-2b/Stentor/Live_ Stentor_1/live_stentor_1.htm |
|
Some confirmatory characters, better observed with a larger power (400x), are the width, the number and the position of the longitudinal bands of ectoplasmic granules, the presence, the number and distribution of rigid cilia (stereocilia) between the normal ones, the size, and forms of the extended individuals.
|
Conditions for the identification Careful
live
study is essential. The best data at weak enlargement (40x and 100x -
objectives 4x and 10x, with an eyepiece 10x) will be obtained on
individuals
fixed on a substrate and extended in their normal attitude of food
gathering. The
specimens can be anaesthetized by mixing carefully in the liquid which
contains
them small drops of a 1% solution of
sodium or potassium iodide, but it is very difficult to obtain a
perfect
anaesthesia in conditions of total extension. The use of
strong
enlargements (400 - 1000 X) can be used only on specimens carefully
compressed
between coverslip and slide. One can resort for this "to the petroleum
jelly compressors". Lubricate the palm of the hand with a very thin
layer
of solid petroleum jelly (Vaseline) and pass on both opposite borders
of a coverslip.
With a micropipette select one or several individuals and drop them in
the centre of a slide. Apply the coverslip over the very small drop of
water containing the protozoa, in
such a way that the drop remains in the centre of the cover. By using
one or two mounted needles, the small quantity of petroleum
jelly will make it possible
to apply and vary the pressure on the specimens, while the
two open
borders will allow water addition, anaesthetics or other reagents with
a
micropipette. If one wishes to make longer observations, use a seal with paraffin, or a microaquarium. Using phase contrast illumination can be useful to
define ciliature and even better to identify rigid cilia, but it does
not appear
suitable for the study of internal organization, because there exists
in the
cytoplasm too many inclusions of very similar index of refraction. It
is
advisable to try the darkfield stops and contrast filters. The
protozoan
must be
observed in all possible positions, as it is free, or as adhered to the
substrate, if it is sessile. Many of these details and especially the color of the ectoplasmic bands of granules, can only be interpreted by microscopists, during the direct examination of the live individual. To highlight in pictures the required identification details, they must be taken at several powers and with a high resolution digital camera (if possible with a 2 or more Mpx), specially focusing on the important details (e.g. ex. stereocilia). |
Consulted Bibliography
- As well as Kahl’s
1935 basic and
irreplaceable work, we
consulted that of Corliss
1961 and Foissner and Wölfl 1994,
which carries out a brief
but complete and informative revision of the genus, by treating all the
species, almost 50,
described after the traditional work of Ehrenberg 1838. They
recognize as valid only 18
of those species.
The Lynn 2002 publication on line, which summarizes the genera and the type species of ciliates is also very useful to see in detail the position of Stentor in the traditional taxonomic system of Ciliatea.
I thank very much Professor Daniel
Nardin, whose kindness
and effort allowed me to
consult the article of Fauré-Fremiet
1936, on Condylostoma
auriculata which
will be much commented on subsequently.
Systematic position of the Family
Below are links providing an introduction
to the taxonomic categories and
traditional hierarchies. If the reader does not have any background on
taxonomy I
suggest they must be your
first reading (at least the
first two).
http://www.niles-hs.k12.il.us/rutgle/Classification.ppt
http://anthro.palomar.edu/animal/default.htm
http://www.ucmp.berkeley.edu/clad/clad4.html
Parts of a specific name
Stentor multiformis (O F
Müller, 1786) Ehrenberg
1838
1
2
3
4
1 - name of the genus (it is always written with a capital letter)
2 - name of the species (one always writes it with small letters)
3 - name of the researcher who described the species for the first time, but probably in a different genus, and dates of the description.
4 - name of the researcher who assigned the species to the correct genus, and year of the revision work
When there have not been modifications of the name since its description one only uses 4, ex:
FAMILY STENTORIDAE
Taxonomic situation
and its
relations according to Lynn
2002 (see the references).
Phylum Ciliophora Doflein, 1901
Class Heterotrichea
Stein, 1859
Order Heterotrichida
Stein, 1859
Family Stentoridae Carus, 1863
However in 1936 Fauré-Fremiet, had already proposed this species, which he described and illustrated in detail from specimens collected at Concarneau (Bretagne, France), is regarded as a member of the genus Condylostoma, corresponding to the family Condylostomatidae, and its criterion has enjoyed more acceptance from the protozoologists, who rejected Condylostentor, created without bringing a valid criticism to the work of Fauré-Fremiet. Consequently Stentoridae continued to be a family with only two genera (one of them very poorly known) until 2002 when almost at the same time two new genera Maristentor and Heterostentor, were described and appear to be accepted for the moment.
|
are consequently
the families closely related
to the Stentoridae. I
want to
propose a key for the reader to recognize the three families. Read
carefully,
because
I include extended comments and some images between the various
options. The key is based on
dichotomous
selections. There are only two options, one is true, the other not. You compare
your specimens with the options and decide which is in conformity with your
material. The option gives you a denomination, or sends you to another
dichotomous option.
B (A)
Body large, (700-900 microns)
contractile; with one undulating
membrane very visible on the right* anterior end;
one Adoral
Zone of Membranelles (AZM)
delimits a peristomial bottom without cilia
................................................................................................... CONDYLOSTOMATIDAE
With one vast
peristome, V shaped; macronucleus moniliform
…………………………..................................................... Condylostoma Bory
de Some species of Condylostoma can be
seen at
Note: Kahl presents
and illustrates moreover S. auricula S. auricula
according to Fauré-Fremiet 1936, and
Foissner and Wölfl 1994 is most probably a species of Condylostoma,
however Kahl allots a single
and oval
nucleus to it. Obviously this species (if it exists) needs to be
newly found and well described. It was found only in 1881 among bryozoa
in
the aquarium at Westminter.
Included here is Fabrea Henneguy 1910, a genus found in brackish water
(up to 20% of salinity), often found in saltworks, and Climacostomum,
Stein 1859,
from fresh and
brackish waters. Species
of the two genera are relatively small (around 300 microns). Image of Fabrea salina HTTP
://power.ib.pi.CNR.it/groups/fabrea/ottico.HTML
|
Now we can go to the Stentor species key

