|
Introduction
This article forms the third part
(Technical
Notes) of the
Key to the Genera of the Bdelloid
Rotifers
already published. Once written I realized that most
descriptions of the micro-habitats, and also the techniques to be used for
each of them, could even be applied almost without modification not only
to Bdelloidea but to many other groups of micro invertebrates
(gastrotrichs, nematodes, rotifers Monogononta, the inhabitants of the
meiobenthos, including the kinorhyncha, and with a few modifications that
surely the imagination of the amateurs will discover quickly, to the
entomostracans and the hydracarina). Consequently I modified the title to
make it more accessible to the indexing for the Internet, although I
maintain the text without change. My primary objective is still to help
stimulate more amateurs' work on bdelloids.
I use here some terms to designate “biocenosis” that
probably are not supported by the European or even American bibliography
(they were coined in Institutes of Limnology from the southern hemisphere,
where the researchers must deal with a much diversified biological world).
I believe that it is very useful to separate which most of the time are
different and identifiable species groups, which could be otherwise
confused if you don’t make a careful selection of the microhabitats. I
make a short definition of the used terms.
Habitat
Leaving alone Zelinkiella,
an exclusively marine genus, which lives as a commensal on holothurians,
the bdelloid rotifers are found in practically all ecological habitats,
although most of the time in freshwaters (or systems that could dry
completely, being periodically dampened by the rains). Only 21 species in 8
genera have representatives which have been found in continental waters,
salty or brackish. (Fontaneto et al, 2008) and the majority are haloxenic
species, that is to say, species that are suspected to have only arrived
and survived by chance in those habitats.
In fresh waters they can, and they must, be
looked for in zooplankton of the larger water bodies (fishing with a fine mesh
of about 70 microns at the most) but there will be found only a few
species mixed with the frequent Monogononta. The most fruitful habitats
are described next.
The classification of the biocenosis is not
capricious. Each one has its own physical, chemical and structural
characteristics, which determine which fauna can live there. One must
learn how to recognize them.
a)
littoral forms:
those that swim
free near the edges of the water bodies, (sometimes called
heleoplankton) or crawl on the littoral plants. Between
these must be distinguished those related fundamentally to
pleuston,(floating plants, like Salvinia,
Pistia or Eicchornia) and those that are
periphytic
(growing on
and around a support) over the
bafon,(submerged part of the rooted
plants). Or the ones bound to plocon
(floating filamentous algae, fixed to the shore,
stones, or objects) and to heteroplocon
(free floating filamentous algae). The
relationships of these faunas, between them and with those free swimming, are
debatable, but in most cases they are distinct species.
b)
inhabitants of other
bioderms (also called biofilms),
thin layer of bacteria, micro-algae and micro-invertebrates that occur on
permanently or temporarily submerged substrates, like stones, wood, the
submerged areas of the floating or emergent plants, submerged roots of
trees, walls, wood, etc. (periphyton).
c)
those that live between the grains of
littoral sands (psammon) or in the thin layer of the superficial
benthos (ooze).
d)
sapropelic, those that live in
ponds and small pools contaminated by organic matter in decomposition
(in any one of the previously described situations).
e)
those that live in waters that are
collected periodically in cavities of trees (tree-holes), or in the
“pitchers” (pitfalls at
the end of leaves) of the carnivorous plants like Nepenthes, or in
the “cup” or “vase”
reservoir, that forms in the center of many
bromeliads, because of a rosette of overlapping leaves
(phytothelmics).
f)
those that live in periodically dried
mosses or lichens (bryophytes), in and under pine needles and
litter (edaphic) or in cavities, be they always humid, or flooded
periodically, on rocks, walls, buildings, pavements, etc.
(lithothelmic).
g)
in addition, there are 3
epizoic genera (Anomopus, Embata, Zelinkiella),
commensals on freshwater animals; (one of them, Anomopus in
brackish water).
It is becoming increasingly common to call
the species that thrive in the e and f situations
limnoterrestrial, because evidently they are in terrestrial environments, but thriving only when they are wet or flooded.
Sampling and
conservation
All trips in search of qualitative
samples of bdelloid rotifers must be specific, directed to a certain
habitat (i.e: periphyton, bafon, etc), and very limited in the sampled
volume. A single live sample can provide sometimes many species, with
few individuals each, which will force a slow and individualized study.
Three or four samples would be the ideal. An ample investigation of a
selected habitat, must be divided into many small samplings throughout a
certain time. This will allow not only a better idea of the
specific richness (the number of species present at
the site) but to obtain a first sight of the possible successive
substitution of the species, driven by physical events such as the seasons of
the year, or the state of humidity of the materials. The live samples
must be protected from abrupt changes of temperature, they may be best
transported in a thermally isolated box.
Of course the sampling methods are as varied
as the habitats that must be investigated, and in addition not only
provide rotifers but a very varied microfauna, although here I have
concentrated on the methods best adapted for bdelloids.
The epizoic rotifers can only be
collected by examining the animals that lodge them and washing the open
surfaces (which include, by example, the cavities where gills of the
crustaceans are sited, or the mantle cavity of mollusks) to gather the
agile or fixed organisms that inhabits them. (Vagile: not fixed, moving
animals.)
The coastal forms raise a true
challenge to the talent of the collector. It is easy to gather, with small
plankton nets, the forms that swim free near the shore, or between the
plants. But there also exists a varied microfauna that adheres more
intensely to the surfaces, or that inhabits more intricate habitats.
For
pleuston, plocon and heteroplocon there is no other
remedy than to gather portions of the corresponding flora, carefully
sliding them with its water and fauna within bags or
bottles.
Periphytic fauna. This is most
difficult to gather. For the inhabitants of the bioderms which cover the
submerged part of diverse rooted plants, stones, wood, etc., one generally resorts to lift off, with care and skill, with the help of
a brush or spatula, the material adhered, directing it (with luck) towards
the harvesting containers. For rooted emergent plants (e.g. rushes) a somewhat safer method is to cut (whenever you can) the aerial
part, inverting a bottle or plastic bag to surround the submerged stem
with its adhered microflora and microfauna, and cutting the plant
below the mouth of the container, closing this and removing from the
water. This even allows advanced researchers a quite exact quantitative
sampling.
For plankton the conventional
plankton nets with 70 or 45 micron mesh are used. They can be towed
behind a boat driven at a low speed. Or the fauna can be fished-out of a
“water column”, lowering the net to the bottom and recovering it
vertically with a slow and steady ascent.
For the rotifers of mosses, lichens,
litter, sands and soil, samples will be taken which must be
investigated later at the laboratory.
The sands must be collected with
abundant water from the sampling site. Shaking them, to suspend them, in a
great volume of water, can help to loosen and suspend the interstitial
fauna. Let it rest some seconds so that the sand settles, and pour off the
supernatant water. Concentrate it by straining through a fabric of 40 to
70 microns mesh. As many of the psammobionts
(animals which live in the interstices of the sand,
at the shore of the water bodies) adhere strongly to the sand
particles, researchers generally try to anesthetize the microinvertebrates
using magnesium sulphate, or bupivacaine, (see “anesthetics”) which
facilitate its removal. It must be investigated with care the
effectiveness and the form of administration of the anesthetics when
collecting bdelloids. The times and suitable concentrations vary according
to the species present in the samples.
Some investigators propose to leave the sample alone
for two days. The oxygen at the bottom will be consumed
and the animals will creep towards the surface. It will be possible to
gather them with a pipette at the interphase between the sandy substrate
and the water. The best site to search is the angle between the sand and
the glass walls of the container. Exploration can be continued over one
week or more.
The lichens and mosses must be wetted
with distilled water or, better, rain water, and they will be let to rest
for two days. They can be squeezed to separate the water from its
microfauna, and the liquids will be investigated during several days with
the stereoscopic microscope or under the low power objective of the
binocular to separate the detected fauna.
The rotifers of ground and
litter are collected by treating the samples in the laboratory, using
a Baermann funnel. This is a funnel with a sieve applied to its mouth,
where the sample is placed. The tip of the funnel is closed with a rubber
tube and a Mohr clamp. The funnel is filled with water up to the level
of the sample. The vagile animals can cross the mesh of the sieve and
accumulate in the rubber tube, from where they will be collected
periodically.
Phytothelmic fauna is better studied
if the plants are carried to the laboratory, if it is possible. Otherwise,
the liquids in the vegetable container must be siphoned to a sampling tube
or bottle and must be transported as any other liquid sample.
Lithothelmic habitats will be sampled,
absorbing the water filling the cavities, and scraping the sediment,
generally rich in algae, and especially in cyanobacteria.
Anesthesia
In the following technical discussion I
refer sometimes to the late H. Taylor. He was a specialized technician that worked
all his life with rotifers, and started his career with the great
rotiferologist F.J. Myers. He published a series of technical articles
that are now bound in a book. Although aimed at the professional
researcher, many of his suggestions are worth trying by the amateur
microscopist.
Those individual fauna selected for a later more
detailed study, or to be mounted as “
vouchers”, (duplicate
specimens, which are filed for future reference and comparison)
will have to be anesthetized, if it is possible.
The best
ever known anesthetic for rotifers, including bdelloids, is the “Liqueur
de Rousselet”, for which we have two formulations, the original, and a
modification due to De Beauchamp. The Italian limnologist Mario F. Canella
says (1954) that even
traces of this reagent were so effective that they stretched immediately
the individuals of Rotaria neptunia.
Even if all
the species were not so collaborative, it is worth the trouble to try the
Rousselet if its basic ingredient can be obtained:
Cocaine Chlorhydrate.
ROUSSELET original
(0.006%)
Cocaine chlorhydrate 2% solution…………………..3
ml
Methyl alcohol (full strength)…………………………..1
ml
Distilled water……………………………………………….....6
ml
De BEAUCHAMP, modification
(0.05%)
Cocaine chlorhydrate………………………………………..0.5
g
Methyl alcohol (full strength)………………………….5
ml
Distilled water………………………………………………....5
ml
With
either of the two formulae it is recommended to add 1 drop of anesthetic
to each mililiter of sample, every 5 minutes, until obtaining the
narcosis.
In addition to the Rousselet formulae, many other
anesthetics have been tried.
Most used now is Bupivacaine (also known as
Marcaine).
Bupivacaine
(Marcaine) was recommended by H. Taylor as a 0,5% mother
solution. It is an anesthetic used by dentists, which I obtained in
presentation of 30 milliliter with 150 mg. of active substance. Dilute it
and use carefully drop by drop, or infiltrate the diluted solution under
the coverslip. It is a proven narcotic with Monogononta, I tried it with
bdelloids, but results were successful only a few times. Segers has
successfully anesthetized Adineta ricciae with this
substance.
1% Magnesium chloride or
sulphate, was also recommended by Mario F. Canella (1954). Also
use it drop by drop. There is some note of its successful use in some
species of bdelloids, but most of his pictures are from Monogononta. An 8%
solution is regularly used by meiobenthologists to loosen the microfauna
clinging to the sand from marine habitats.
Neosynephrine (Phenylephrine) ophthalmic
drops are used by ophthalmologists to dilate the pupil of the eye.
They are also sold as nasal sprays. You must take care with these, because
the drug is very addictive. Doctors recommend not to use it as a medicine
for more than five days! I had tried it a long time ago, and it was very good
(as always) with Monogononta. It was deceptive applied to
bdelloids.
Homatropine can be obtained in drug
stores as a medication for liver diseases, and also as ophthalmic drops.
This, and a lot of other drugs as atropine, benzamine
hydrochloride, xylocaine (lidocaine), tetracaine,
metoprolol, etc. were recommended to be tried. But no one reports
today an always successful anesthetic for bdelloids. But all of them merit
a trial.
The anesthesia must be judged by the
lack of response to the contact stimulation, because cilia do not
anesthetize generally, and the rotifers continue moving, even if they are
well anesthetized.
Fixatives
Sometime ago the anesthetized rotifers
were fixed adding to the containing drop a similar volume of 8% neutral
formol, or 6% glutaraldehyde (H. Taylor used it as a 2%
solution). Formol is now
banned as carcinogenic, but glutaradehyde is not. (See footnote, added
March 13th). Although bibliography shows that
professionals continue to use both products, even if many laboratories
have changed to less toxic fixatives.
GALA 60 (Dioni)
was used in Italy by Dra. Ulrique Uehliger at 10%
concentration, in planktonic rotifers samples, and they reported a
good behavior in samples conserved in the fixative for two months, (of
course most of the species were Monogononta). Nevertheless
it is highly advisable to discard the liquid of the sample, and
wash it with 70% alcohol after no more than a week, and preserve
it afterwards in 70% alcohol plus a 5% of glycerin. This has three advantages: it prevents
the possible corrosion of organisms by the strong acids, provides a
permanent preservation medium, and facilitates the later work and the
faunistic counts eliminating the irritating fumes of acetic
acid.
As the specimens (fixed
or alive) are selected from the sample being searched, they will be
withdrawn with a micropipette and accumulated in a small watchglass or any
small capsule, to conserve them for future study.
Although it is always advisable to bring a
live sample to the laboratory, at least in the beginning of a project,
most of the time the samples will be generally fixed in the field. If
bdelloids are the objective, the traditional methods of fixation will show
only unidentifiable contracted units.
So, I propose to divide the sample in
three:
A subsample will
be anesthetized with Bupivacaine (or the anesthetic that would be finally
selected by experimental trials) until it only shows ciliar activity, and
the affected animals do not contract. H. Taylor suggested, as a standard
technique, to split the concentrated sample in 5 or 6 subunits of 4 to 5
ml each, and to apply to each one an increasing number of drops of the
anesthetic, expecting that one turns to be optimal. He also says that a
uniform time (9 min. max.) would be used, to fix the samples. It
seems that more time allows for the specimens to contract even if they
were already well expanded. At this moment one will add to each
subsample, 10 to 20% GALA, with or without any in toto stain. Before, I
used 0.2% Rose Bengal, but it is now considered strongly carcinogenic. Try
the use of much diluted Allura Red, or Tartrazine (respectively red and
yellow food colorants), or, even better, 0.5% to 1% aqueous
Eosin.
The second can be
treated with CO2 (mixing with the sample some gasified commercial water)
or by asphyxia (small flask, concentrated collection, very tight sealing),
which, in this material, can rarely produce some anesthetized
bdelloids. If the rotifers do not die
stretched by the boiling water, there would be certainly many other
invertebrates that do, which could also be very interesting for
the microscopist which for this reason could be prone to do a more
complete inventory of the sample.
Third unit - The
samples gathered by filtration of the sediment detached from pleuston or
bafon, or gathered as benthonic ooze, are generally too voluminous for an
individualized treatment in the field. In this case the best strategy
(apparently designed by Frank Myers) is to place the sample in a container
of a volume 6 times greater, located as a safety precaution, within
another one 2 to 3 times bigger. Time is allowed, so that individuals
stretch out and retake their normal rate of activity, at which moment, 3
to 5 volumes of almost boiling water is poured into the first
container. Sediment is allowed to settle for a long time and upper liquid is
poured out to the maximum possible. A volume of fixative similar to the
sediment volume is added to the sample. As in the second suggested
treatment only a few species of bdelloids respond to this treatment, which
is normally very useful with Monogononta.
Alternatively, the
filtrates or sediments can be placed in a “detachable-neck flask”, with
the base painted in opaque black (Dioni). After a time, the
geotrophic negative, and phototrophic positive, swimming animals, plus those
that suffer with the oxygen rarefaction, will meet in the detachable neck.
This one is taken apart, its content is poured in the definitive
container, and the fixative is added; or the microfauna is first massively
anesthetized before to fix it, or it is treated with almost boiling water
or almost boiling fixative.
Methods of
study
The sample is first studied with a
stereoscopic microscope, or the low power objectives of your microscope.
Preferably over a dark background. And the individuals whose morphology
must be specially studied, or which must be identified taxonomically, will
be separated, using micropipettes with buccal or mechanical control…… (if
you try your pipette under the low power, remember that the movements has
its directions inverted. If your microscope allows it, use the “Dioni’s
poor man stereoscope”. Some investigators consider that
pipettes allow the adhesion of the animal to the glass, preventing them to
be unloaded to the slides, and suggest to learn to manipulate the
rotifers, even live, exclusively with micro-loops, micro-spatulas or
micro-needles. They are saved in
watchglasses, or suitable
capsules of any type, where they are accumulated, and are later
transferred to slides in a drop of water, and first studied uncovered, to
verify its behavior in an unrestricted medium, style of swimming,
etc., or they are covered with a coverslip. As water under the coverslip
evaporates the weight of the coverslip can apply pressure on the
specimens. If this is not prevented the rotifer could be squeezed. To
avoid this
- For voluminous species some support must be
included (paper, cellophane, small coverslip parts, hairs, etc).
- A more technical solution, but a not so
simple one, is to create a petroleum jelly
compressor.
Place a small drop of water, with the specimens to be
studied, on one slide. Spread a very thin layer of petroleum jelly on the
palm of your hand, and slide over it two opposite edges of a coverslip, to
gather a thin line of jelly on them. Invert the coverslip and, carefully,
lower it VERTICALLY over the drop, watching to keep it centered. With the
aid of two thin and blunt needles, (or even toothpicks), adjust the height
of the coverslip, looking at the preparation with the stereoscopic
microscope, or with the low power objective of the binocular, until a
delicate compression of the rotifers is reached, that will be controlled
with great care, to avoid destroying them.
Individualized processing:
a) The selected living individuals, can be
treated with some anesthetic, if any one is useful, fixed with
GALA, to be stained and mounted in permanent preparations, as described below.
b) The individuals of the species already
fixed in the field, which would be needed for further study, must be
separated and collected in a small capsule. If they have been massively
stained with Tartrazine, or Allura Red or Eosin, they go directly
to the glycerin as it is explained below. But, if not, 0.5% Eosin will be
added to them letting it act for the necessary time. By means of
micropipettes, or working with microloops or microspatulas or even
microneedles, the animals must be worked out of the staining
solution, and they will be washed in water, to be mounted in
glycerin.
Glycerin will be applied through several
graduated steps of 10, 25, 50, 75 and 100%, a few minutes in each one, or
they can be placed in a 5% solution that are left to concentrate under a
dust cover, over several hours. The goal is to avoid the specimens
wrinkling, but to concentrate and mount them in pure glycerin (H.Taylor used
glycerin with a “touch” of phenol, as a bactericide and an aid in
clearing). Apparently the fragile species that wrinkle during the mounting
process can become suitably hardened if they are previously treated with
10% acetic acid.
The mastax is indispensable to identify the
species, even if its general structure is very similar throughout the class.
A solution of 0.3% of commercial sodium hypochlorite (supermarket cleaner
bleach) is recommended by H.Taylor. The commercial solution is normally a
5% solution. Dilute 1 drop of commercial solution with 8 drops of water (9
final drops). This gives a 0,6% solution approx (5%/9 = 0,6%) Add 1 drop
to another water drop with the rotifer, the working solution is now 0,3%,
and it starts dissolving the cells and leaving only the hard structures. It
is better to work with a well slide, and with small drops to prevent
losing the tiny structure.
It is also possible to work under the
coverslip, adding a 0.5-1% solution drop to the cover margin and absorbing
with great care the water at the opposite margin with a thin but long
strip of filter paper. It is also possible to use the same technique to
replace the water by glycerin which has a better refractive
index.
ELEMENTS FOR THE DESCRIPTION
OF THE SPECIES OF
BDELLOIDEA
Put your sample in a drop of
water on a slide. Ricci and Melone (2000) suggest that, at first, you study
your material without applying a coverslip. This is easy with the low
power stereoscopic microscopes, but only for the 4x and 10x objectives
with the compound microscopes, and with more or less quiet animals, but
could give you a good appraisal of the general morphology and activity of
the rotifer.
The general morphology will be
described from observations with a 10x eyepiece and the objectives 4x and
10x, with complementary details with a 40x, and some details (the trophi
as an example) will even be recorded with the 100x Oil Immersion
objective, if you have one. The law here is to “document all that is
possible, in the best way which will be possible”.
Digital pictures are a rapid
recording technique. Now you can resort to one of a wide range of digital cameras,
from the “big names” valued at many thousands of dollars, to the most modest
webcams. In Europe Philips is the preferred trade mark, replaced by
Logitech’s in America. Be cautious and do not acquire for your microscope
any camera with less than 1.3 megapixels. Study with care the many articles
on this theme published in Micscape. If you can read French, a detailed
view of the posts in the Forum Microscopies (see the link in Micscape front
page), could be of your interest, long theoretical discussions and
detailed camera presentations has been published. You can work without a
photomicrography camera, even
if one of them can make things more easy for
you. But if no camera at all, you can draw, as hundreds of rotiferologists
did fruitfully in the past. They study and document with drawings and
descriptions almost all the actually known species.
If the rotifer is more or less
immobile, take the needed images to later compose a whole body mosaic. In
all the cases it is highly useful to make z-arranged stacks, open the
pictures in the desired order, and using the Screen Grid function of your
image processor, make detailed drawings, even if some of
them are only sketchy
ones. When you have a clear concept of the
relationship of the organs, you can select and arrange the images of the
stack to apply CombineZ (in any of its later versions) or to use instead
Helicon-Focus.
When studying a new specimen it
could be useful to adhere to the following list, (It is not exhaustive,
even if in many cases could be excessive).
Write and draw all that you can,
you will verify that this is a very useful approach. Don't forget that,
with every note, drawing, or picture, you must record the date and, if it
could be important, the hour. Record also the microscope, objective and
illumination technique, the technique used to record the data, any other
instrument involved. Put a scale on the picture or drawing now. Not
a general statement about a group of images, but an individual scale. You
don’t know when, or what kind of phenomena can hit tomorrow, and separate
you from your materials and notebooks. Record all you can do for your own
benefit, or to make your laboratory work comprehensible to other
scientists. And don’t worry about the word. If you work seriously along
this proposal, what you are doing is SCIENTIFIC WORK (yes, let stand the
capitals) even if you don’t have the ultimate tools to do the
publications, or you don’t work on “The Big Problems” of Physiology, or
Evolution, your contribution to taxonomy and zoogeography will be
recognized and stimulated. These two areas are those which need more of
the push up that the amateurs can give. Many, many years ago, Huxley
states that “Taxonomy is in the hearth of Biology”.
General
data
First of all register the origin
of your sample, describe the habitat in your notebook, record, if you can,
latitude and longitude (NO, now you don’t need complicated apparatus, nor
even expensive topographical maps. You need to use your FREE
“Google Earth”?) In some cases and in many countries you can even include
in your files one or more screen captures to record the locality. Record
any data you can obtain about the source (this includes of course physical
and chemical data if you can) …But, surely you have your computer, your
word processor, your Photoprocessor, your digital camera and your Google
Earth. But a kit of chemical tests, even for aquarists, is not in the
standard equipment of the amateur: search in your wallet if you can afford
the expenses, this
would be a great addition to your lab. Use your digital camera to file
some pictures of the site. They are invaluable aids today, and especially
month or years in your future.
External
anatomy
It is necessary to describe
their appearance and activities when eating, crawling and
swimming.
Body structure - Verify
the shape, in dorsal and lateral view, and the relationship between the
pseudo-segments. Measure the length of the foot, trunk, neck, and the
segments of the head.
Cuticle - Write down the
appearance and thickness of the cuticle, and the presence of folds,
longitudinal furrows, or reliefs. If they exist, note the number,
disposition, form and size, of the cuticle thorns, or any projection,
warts, or papillae they have.
Head and corona - Verify
the form of the head, its width, and the shape of the “corona”, its size
with respect to the head when they are displayed, the order in which the
trochas opens, pedicel length, and its mobility.
Superior lip - While the specimen
eats, verify in detail the shape of the superior lip, especially in
dorsal view.
Antenna - Verify the
position, form, and length of the antenna (in lateral
view).
Eyes - Verify the
presence or absence of eyes, color and position (on the brain or at the
end of the rostrum or proboscis, or at any other
situation).
Foot: number and shape of
segments, form and details of union with the trunk.
Spurs: Shape and length,
insertion on the foot, and width at their base, separation among them, and
any mark that they have.
Toes: number, and
disposition. Absolute and relative lengths, among them and with the spurs.
(It is better to study the bdelloid in a hanging drop, over an o-ring or
well slide. It sticks to the coverslip and one can see much better
its foot and toes, or any other adhesive system it
has).
Adhesive disc: (if
present) position, orientation, structure.
The study of the foot could take
a long time, and a lot of effort, most of the time it is hidden between
algae or detritus. A very fine pipette to isolate some clean specimens is
a must.
Internal
anatomy
Position, form and size of the
brain
Form and disposition of the
sub-cerebral glands and the retro-cerebral
sacs
Mastax: position, form
and size
Trophi: form and size of
each piece. Teeth, dental formula (100x)
Gastric and salivary
glands: aspect, size, position.
Stomach (Intestine):
form, size disposition presence or absence of a lumen, (it can be
necessary to verify it at 400x in some compressed individuals). Only in the
Habrotrochidae there is no lumen
Intestine (rectum):
location, size.
Feces: if the opportunity
allows their observation. In Habrotrochidae the feces are pelleted, similar
to the gastric content, in the rest of the bdelloids they are a loose
material
Protonephridia: 40x and
100x; compressed; they could be easily overlooked, look for the flame
cells.
Bladder: shape, position,
size, relationships. Time for repletion and voiding?
Foot glands: also called
cement glands (number and disposition) 10 and 40x with extended
foot.
Gonads
(germovitellarium): made from two syncitia:
Vitelogen, The big nuclei
that are normally seen even at moderate powers.
Ovaries (Small nuclei
between the nuclei of the vitellaria. They are only well seen in
compressed individuals.)
Oviduct: one, common to
both germovitellaria, difficult to see or to identify.
Developed eg:, presence,
size (in oviduct).
Uterus (in the viviparous
species): mostly indistinguishable.
Position of the opening of
the cloacae: Shape of the near segments if they have any interesting
characteristic.
Egg (layed) - Form, size,
type of covering and any sculpture on the surface.
If it can be recorded:
time to eclosion. You must separate some females into a solution innoculated
with bacteria,
with very few gross particles, in a capsule protected from evaporation,
and record the laying time, the egg structure and the time to
eclosion.
As far as is possible all the
studied details would have to be photographed.
Probably the specimens that are
used for the complete study do not survive the
treatment.
If pictures are not
completely satisfactory (as rarely they are) one can make drawings based
on them, using as a guide the screen grid which can be displayed over the
picture in almost all the image processors. Complete your drawings with
details from live specimens
Some times ago I
published in Micscape an article on the utility of the drawing for the
microscopists
http://www.microscopy-uk.org.uk/mag/artsep02/wddraw.html
If a specialist can
appraise most of the pictures, drawings, notes and measurements here
advised, (and it would be a lot of good information) they will have very
little difficulty to classify the material, even if this could be new to
science.
THIS IS WORK, HARD
WORK, BUT WE ARE SEARCHING ALL THE DAY FOR A GOOD SUBJECT TO BE SEEN AND
RECORDED WITH OUR EQUIPMENT. HERE IS A GOOD OPPORTUNITY TO BE HAPPY AND
REALLY USEFUL.
Present
situation
Claudia Ricci and Giorgio Melone
in their work of 8 years ago (2000) hoped that their paper would wake up the
interest of amateurs and professionals on Bdelloidea.
The reasons that prevented a
fast concretion of this desire are also explained by the authors: small
size, excessive mobility, lack of useful media to slow or anesthetize
them, incredible speed for total contraction, difficulty to stop them by
compression without badly deforming them, necessity to study the
individuals for hours, alive, to be able to decipher their
organization.
The books with suitable keys and
descriptions are mostly out-of-print editions, and it even influences
negatively the fact that outside Europe few students have a good knowledge
of German, because the fundamental work on Bdelloids is
Donner. J. 1965. Ordnung Bdelloidea.
Bestimmungsbücher zur Bodenfauna Europas, 6, 297 pages.
This last difficulty could be
partially resolved if this book is translated to English and or
French.
This illustrated Key, which
we include today in the Micscape Magazine tries to make available to
students, in direct visual form the morphological basis that allows separation
of the Bdelloidea genera.
It is a pity that I cannot
include a picture of each genus, When I found them I took some drawings
from Internet. But even then there are some for which I could not
find images (I live far from the scientific libraries, and on the Internet
there are not many images).
But it will continue being true
that their does not exist a sure medium to anesthetize the Bdelloids. Apart
from
Rousselet's, any tried technique gives as a most probable result the
contraction of the rotifers so fast and complete that it disqualifies any
further study, except for the recovery of the trophi by dissolution of
soft parts with hypochlorite. In this Class the trophi are not generally a
very important specific character, mostly confirming, better than
defining, the determination. But the Italian team at the University of
Milano is assembling an important library of trophi images captured with
the SEM and this could change things in the future.
Nevertheless there is a tool
that surely will facilitate the
investigation of the species of Rotifera. The professionals of course, but
now even many advanced amateurs, have incorporated to their equipment not
only the powerful Nomarski DIC microscopes, but also the photography with
electronic flash.
Figure 2, of the first part,
which we reproduce below by courtesy of its author, Charles Krebs (who
with it inaugurated a new approach that surely will attract the efforts of
many other amateurs) illustrates the magnificent results that augur this
technique. We add other pictures by Krebs and Abel Lear, and others shown below, which
confirm that.
The availability of flash, digital photography in hi-res, and the now popular programs for
three-dimensional reconstruction (CombineZ5, CombineZM, Combine ZP, and
Helicon Focus (a and b methods)), augur an important generalization of the capacity to
investigate and to document this difficult group of
microinvertebrates.
Amateurs have an important
opportunity here to collaborate with the professionals (who have the
suitable bibliography, and the scanning electronic microscopes) sending to
them high quality descriptions, measurements, and detailed photos of the
species they observe. And this can be made on a world-wide scale thanks to
the aid of Internet overcoming the geographic barrier.
I hope that the insatiable
curiosity of present and future members of the forums of microscopists,
will produce an ample harvest of documents to make them available to the
specialists.
Nevertheless, it is clear that,
due to the exigencies of bibliographical availability, and access to
electron microscopes, the specialists in the Universities and Research
Institutes, will continue to be the ones who can identify with certainty
the species of bdelloids, and while the number of those does not increase
substantially, and the photomicrographic documents harvested by amateurs
don’t run fluently: “The biogeography of the Bdelloidea, (also in
general for all Rotifers – author’s note) will continue to be,
really, the biogeography of the students of the group.” (Ricci and Melone,
2002)”
To facilitate
the contribution of amateurs to this discipline I add at the end a list of
the more active specialists I know. I think that most of them would be
glad to receive taxonomic novelties from all over the
world.
SENDING
SAMPLES - If the amateur does not feel
himself able to accomplish the more difficult tasks, he or she can make a huge
contribution by sending samples to the specialists. Remember you are working
with species that (almost all) can survive after desiccation. Using a
piece of laboratory filter paper (or coffee-makers filter paper) you can
prepare a sample that could withstand the hazards of even the surface
mail, and can be included in any mail envelope. THIS IS SIMPLE AND
VALUABLE.
But remember that a researcher
is a very busy person. Take care to contact first the specialist of
your election to ask for their permission to send your materials,
pictures and dehydrated samples.
If after seeing
your notes, pictures and drawings, the scientist tells you that your
material is of real interest, prepare a desiccated sample to send her or
him some specimens.
How to prepare the
sample
Concentrate as many as you can
of the individuals in a little volume of water. Prepare a Petri dish, or a
similar flat dish with a cover, with a piece of filter paper on the
bottom. Lightly wet (not flood) the paper with some drops of distilled
water. Deposit the sample with the living rotifers in the center, and
cover, letting a thin open gap to allow evaporation. Dryness must be
attained in 4 or 5 hours. Cut the dry filter paper to recover the area
with the sample and put it inside a folded paper. Include this with the
letter you send with data. Don’t use plastic for the holding paper or the
envelope. There is a good chance that the individuals in your sample can
be easily recovered in the destination laboratory.
Even if you
don’t result being the happy parent of a new species, your sample could have
zoogeographical and ecological interest. If you are doing that you are a
very careful amateur, and of course you know that a full series of data
must be sent with the sample.
BIBLIOGRAPHY
Jersabek, C.D., H. Segers, and P.J. Morris,
. An illustrated online catalog of the Rotifera in the Academy of
Natural Sciences of Philadelphia (version 1.0: 2003-April-8). [WWW
database] URL http://rotifer.acnatsci.org/rotifer.php
Diego
Fontaneto and Claudia Ricci – 2004 – Rotifera:
Bdelloidea, in Fresh water Invertebrates of the
Malaysian Region.Pg 121-126
Edmondson,
W.T. – 1959 – Rotifera. Pages 420-494 in W.T.
Edmondson (ed.), Ward and Whipple’s Fresh-water Biology, Second
edition. John Wiley and Sons, Inc. New York, NY.
Fontaneto
D., Boschetti C. and Ricci C. – 2008 – Cryptic
diversification in ancient asexuals: evidence from the bdelloid
rotifer Philodina flaviceps. J . Evol. Biol. 2: 580–587
Haigh
S. B. 1963 - Notes
on the study of bdelloid rotifers. Quekett J. Microscopy, 29: 133-138.
Hendrik
Segers – 2007 - Annotated checklist of the rotifers (Phylum
Rotifera), with notes on nomenclature, taxonomy and distribution.
Zootaxa 1564- Magnolia Press
Hyman,
Libby H. – 1951 – The invertebrates –
Acantocephala, Aschelmintha and Entoprocta, McGraw-Hill book Co.
Ricci
C. and Melone G. – 2000 – Key to the identification
of the genera of bdelloid rotifers. Hydrobiologia 418: 73–80.
Ricci
C., Melone G. and Walsh E.J. – 2001 – A carnivorous
bdelloid rotifer, Abrochtha carnivora n.sp. Invertebrate
Biology 120: 136–141.
Claudia
Ricci, Manuela Caprioli and Diego Fontaneto – 2007
– Stress and fitness in parthenogens: is dormancy a key feature
for bdelloid rotifers? BMC Evolutionary Biology, 1-7(Suppl
2)
Ricci
Claudia y Giulio Melone – 1998 – The
Philodinavidae (Rotifera Bdelloide): A special family. Hydrobiología
385:77-85
Ricci
Claudia, Russell Shiel, Diego Fontaneto & Giulio Melone–
2002
– Bdelloid
rotifers recorded from australia with description of Philodinavus
aussiensis n.sp.
Bdelloidea
specialists: In view of the possibility of annoyances to the specialists
by mechanically sent spam, finding their e-mails addresses is left to the
initiative of the advanced amateurs. Communication
methods are mentioned in their bibliography, and most titles are published in Internet in PDF
format.
Caprioli,
Manuela (Italy)
De Smeth,
Willem (Belgium)
Fontaneto,
Diego (Italy, UK)
Melone,
Giulio (Italy)
Ricci,
Claudia (Italy)
Schmid-Araya,
J.M. (UK)
Segers,
Hendrick (Belgium)
Song,
M. O. (S. Korea)
Sarma,
S.S.S. (Mexico)
Images
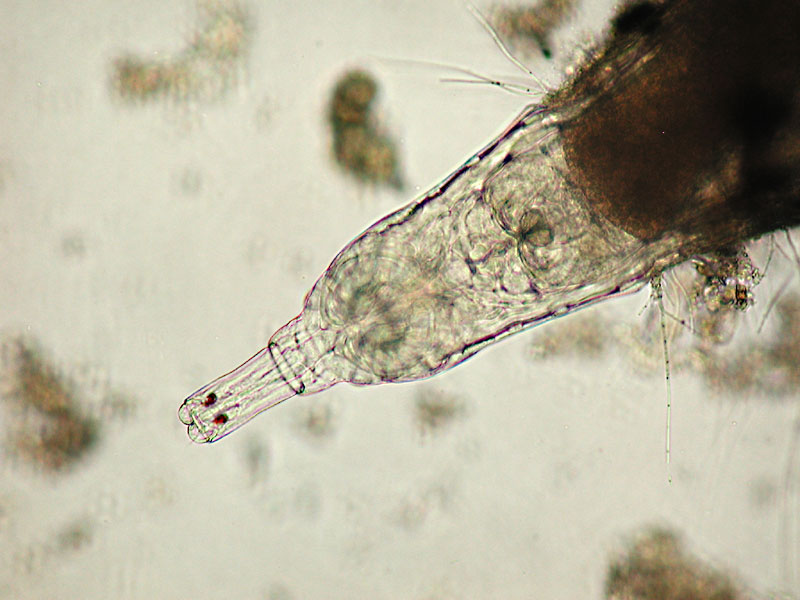
1
– eyes at the end of the rostrum in Rotaria, bright
field – Oliver Barth

2
– head of Philodina – electronic flash –
Charles Krebs
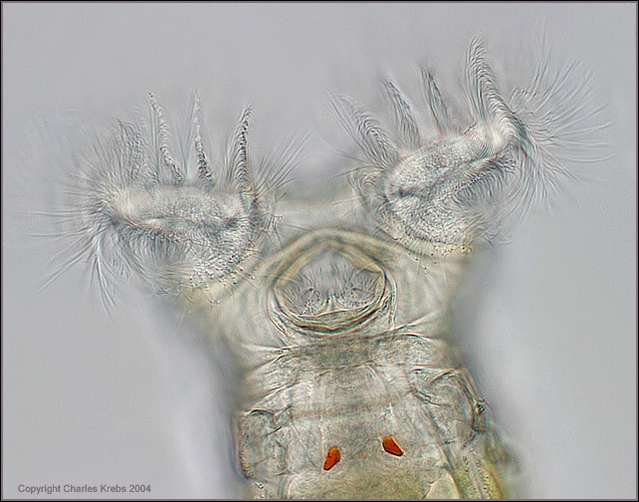
3
- another head of Philodina – bright field, electronic
flash, by Charles Krebs, showing the red eyes
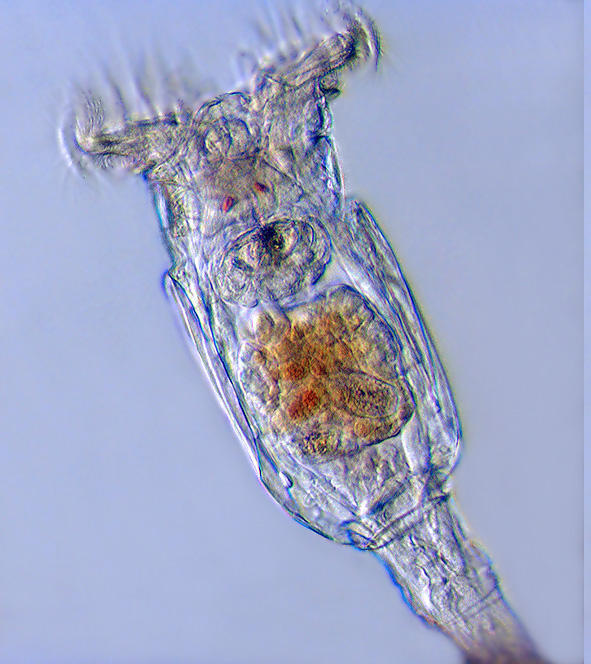
4 – Philodina megalotrocha –
DIC – a picture by Abel Lear
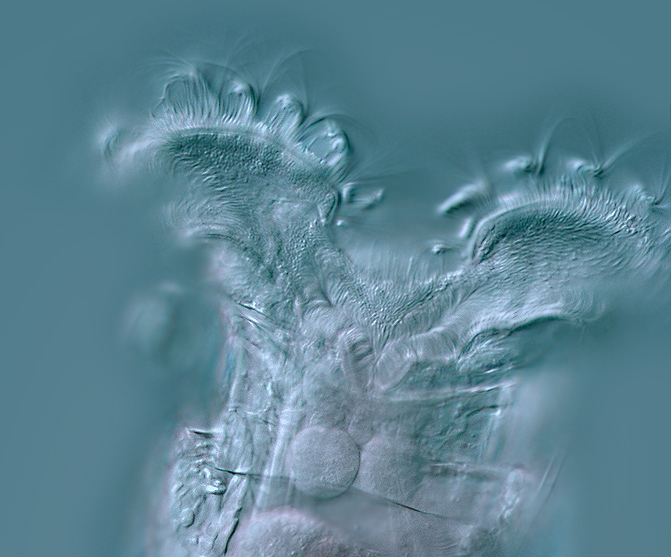
5
– Another DIC image of the head of a probable Philodina, by
Abel Lear
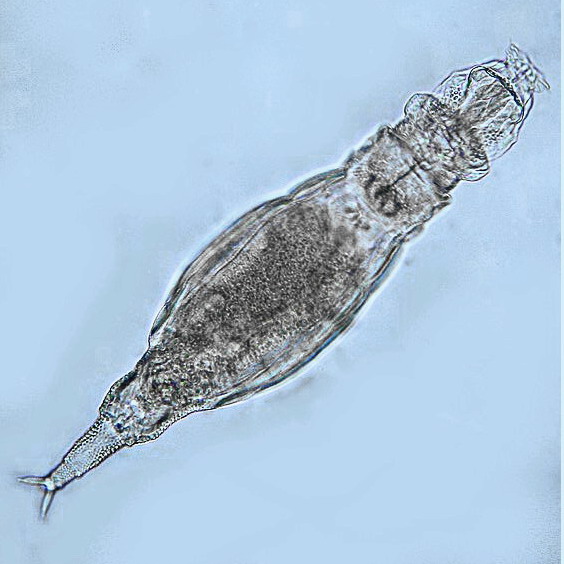
6
– Adineta cf. tuberculata, bright field, Michel Verolet
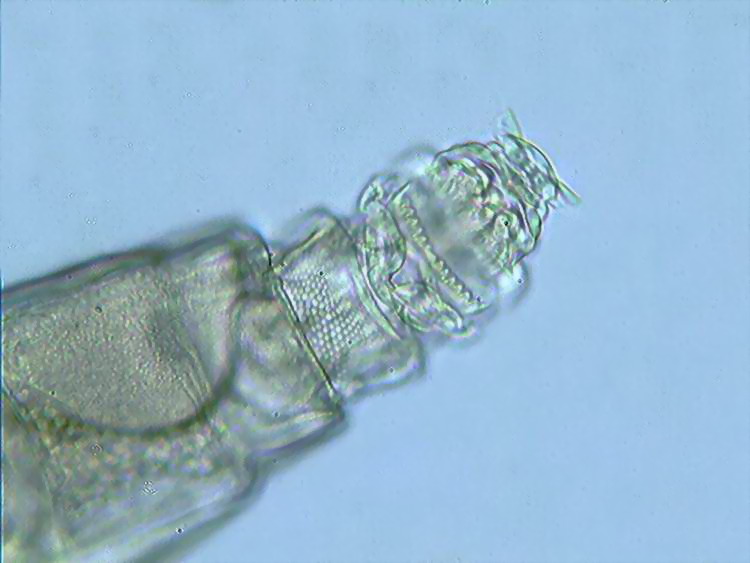
7
– a view of the head of A. cf. tuberculata, by Michel
Verolet
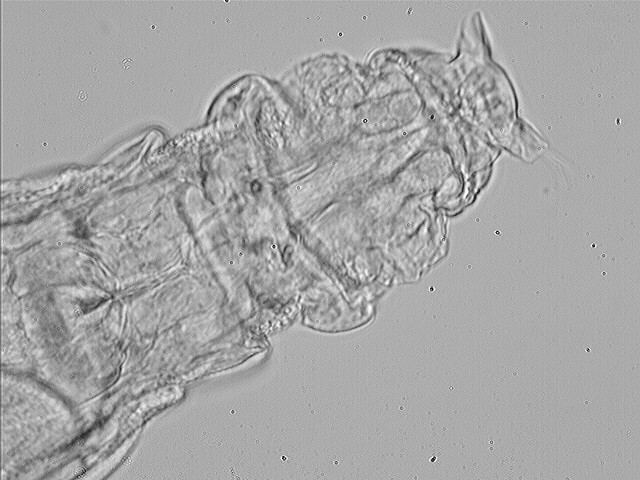
8 –
dorsal view of the head of the same, bright field, Michel Verolet
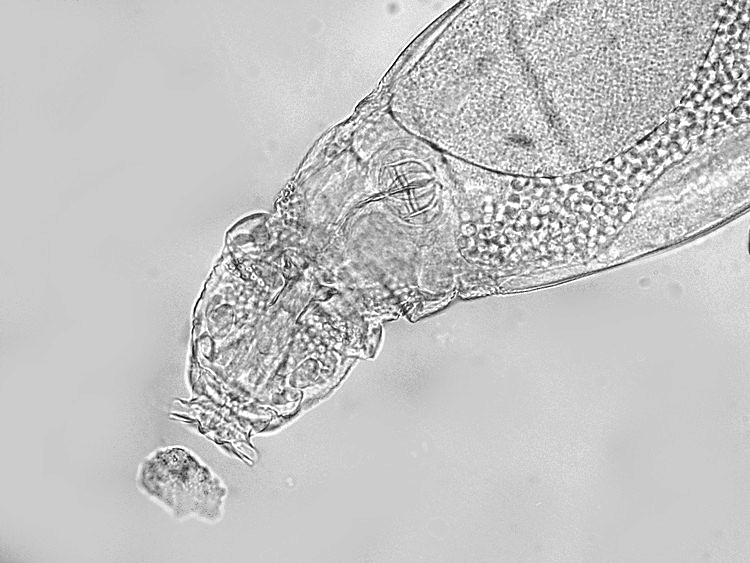
9
– bright field at its best, detailed anatomy of the head of
Adineta, showing the complex rostrum
Michel
Verolet

10
– The new megapixel digital cameras allow detailed pictures of
active animals, and even movies at a good resolution. Adineta, by
M. Verolet
11
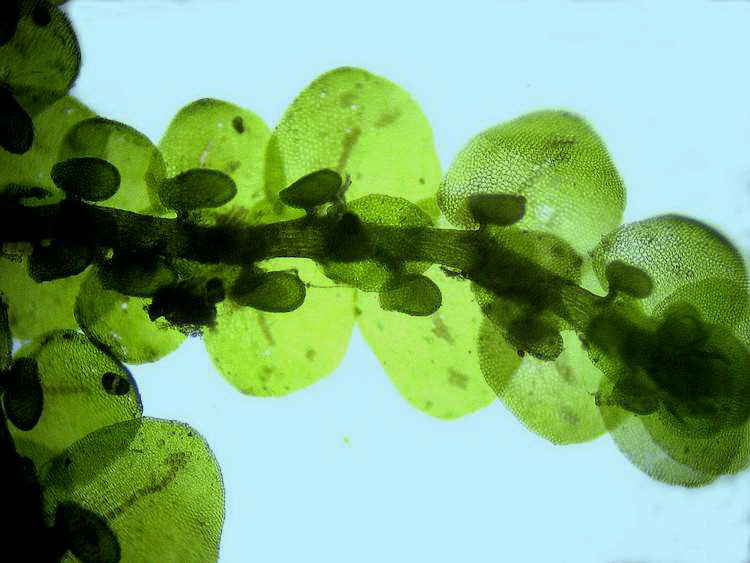
– Habrotrocha attached
to a bryophyte, picture by M. Verolet
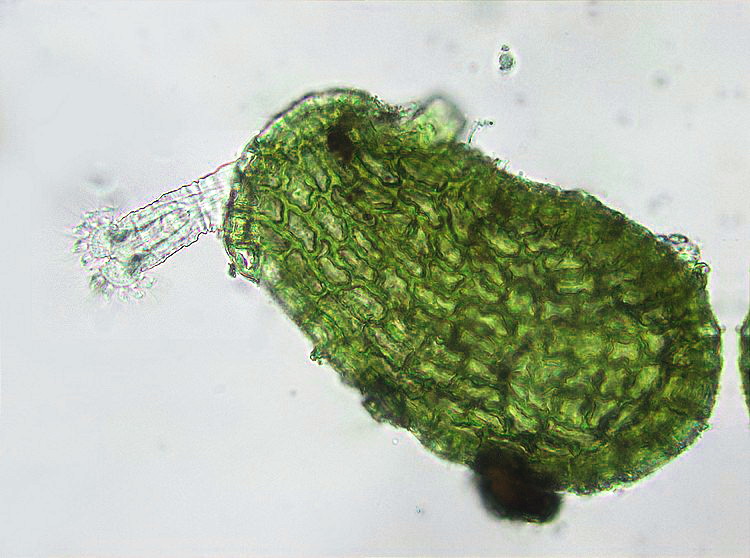
12
– one of the attached examples - a stretched Habrotrocha, M.
Verolet

13 –
Rotifer’s eggs glued to an algae filament, M. Verolet
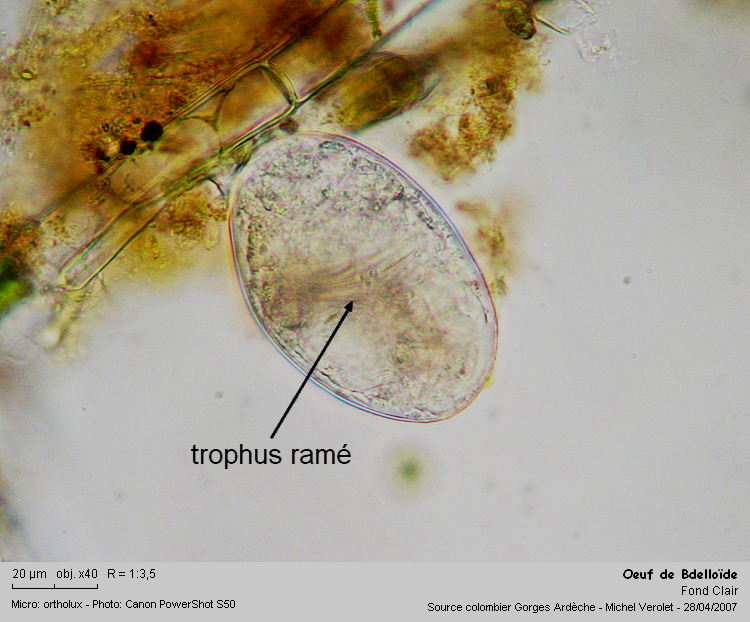
14 –
One of the eggs, the ramate trophi confirm it is from a bdelloid
|














