|
INTRODUCTION
Of all the products
used to prepare microscopic materials for their definitive mounting on
slides,
the dyes are the more difficult ones to be substituted. Their selection
has been made over decades by specialized chemists and microscopists who have chosen
with exquisite care the products suitable for each task. There is a
delicate
chemical balance between the stain and the organelles or
cytoplasmic
products that they are intended to color.
After more
than 100 years of experience, any proposed substitute is destined to
be third rate, as opposed to the professional dyes, and many of these
are
absolutely irreplaceable. For more information search the Stain File (below).
Most
amateurs understand this, and, when really in need, if they can,
they take recourse
to the most common classic colorants as Hematoxylin, Eosin, Carmine,
Methylene
blue, Methyl green, Fuchsine, and the like. The big problem is
to acquire them
in small quantities and at reasonable prices. The Europeans have at
least two
resources (listed in the
appendix).
Nevertheless,
the young person, and sometimes the not so young amateur microscopist, may wish
to highlight nuclei, cytoplasm, cilia, cirri, plastids, and
membranes to
study them easily but without setting out, of course, to make a
professional result, and to prepare professional formulae.
It is for
them for whom I have written up these notes.
|
This is an
impossible picture. It is an illustration mixing picture
elements,
enhanced with a little design when needed. It was composed in
PhotoPaint to show the most abundant inhabitants in my
microscopic jungle of Cladophora.
|
MATERIALS
and METHODS USED
Three years ago
before moving to Durango, I wanted to keep some samples from
the very varied microfauna that lived on or between a Cladophora tuff that
inhabited my freshwater aquarium at Cancún. And I took the opportunity to try a pair
of dyes and a probable mounting medium whose possible utility
intrigued
me.
Cladophora is a chlorophyte with a
thin and profusely branched filamentous thallus
that sometimes invades like a plague the aquariums of the amateurs. My
aquarium
was not an exhibition aquarium but a source of biological materials, so
the
alga grew on the understanding that it had the right to balance its
own development with the many
other inhabitants of the aquarium which included a numerous population
of
guppies. These and their abundant young passed their time exploring the
tuffs of weed
probably to feed on the microfauna that lived between and on it, and also on the new growth of alga.
In order to
obtain the required sample, I introduced underwater a small part of the
algae into
a plastic tube (discarded from a photographic 35 mm film) I cut the
contents and
I removed the tube.
I filtered the
water, without applying any
type of anesthesia, covering the mouth with a piece of silk
screen with
a mesh of 70 microns, and I filled it with hot AFA,
a fixative with alcohol, formalin and acetic acid.
Unfortunately
the fixation (and dehydration) squashes the thalli and turns them into
unattractive flattened ribbons but it preserves very well the microfauna.
COLORATION with ALLURA RED
| Note
on the dietetic dyes: practically all, including Red 40 have
been accused of being
carcinogenic, although their morbidity incidence is very low. There are
two
types of dyes, the water soluble ones, which are normally sold to color
cakes,
ice creams, etc. And the alcohol soluble (called lakes) and which are
generally
sold as a dust, for commercial and industrial use. The ones I used were
the
simple water-based solutions sold in the food stores for domestic use. In the
text and in the appendix I include the USA and EU
codes for the
cited dyes. |
I washed
out the fixative with 30% alcohol, and finally with demineralized water,
and after
discarding this, always making use of the silk mesh, I filled
the tube with
a solution of Allura Red (a dye which in the U.S.A. has the FD&C
Red #40 code,
and which is known in the European Union under the E129 number). The
Allura Red
is a dye approved for foods and sold in the form of a 2.8% aqueous solution.
The
commercial solution is extremely concentrated and I used a dilution of
6 drops
of solution in 30 milliliters of water. I leave the materials in
contact for 3
hours. (Probably one hour could be enough.)
The
following treatment included the washing of the dye with water (two
changes)
and a progressive dehydration with 30%, 50%, 75% alcohol, (half an hour
in each one)
and 96% (of this last two changes, each of one full hour).
It is very
probable that the dehydration protocol has been excessively long, but
the
behavior of the nail enamel that I thought to use as a mounting media
was unknown
to me and I preferred to err on the safe side.
MOUNTING IN NAIL
POLISH ENAMEL
I placed a
heavy drop of nail enamel on a coverslide. I passed a piece of alga to
the
center of one slide, I extended its
branches
with a pair of needles, and removed with an absorbent paper and very
quickly
most of the surrounding alcohol and turning
down the coverslide I lowered it taking care with the material.
I put the
preparation safe from dust and under a 10 g weight (a flat headed screw
of 6 mm diameter by 2.0 cm. long, with two nuts screwed on) for 10
hours. On
the following day the slides were hard enough to allow safe use even with
the immersion objective. As a precaution I made an additional seal
with the
same enamel.
The mounting
was extremely successful, and supports my
recommendation for the use of the nail enamel as a synthetic
resinous
medium at least at the amateur level.
The
preparations are now more than 2 years old and as it will be seen from
the enclosed images they
perfectly maintain the morphology of the subjects, and also
retain the color of dye used.
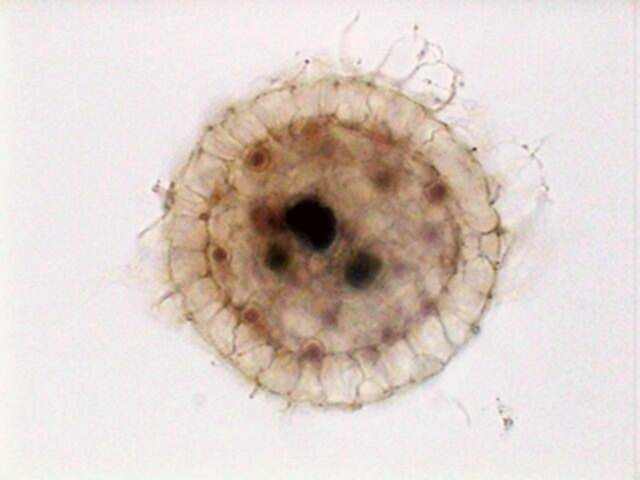
Most of the
protozoa that I found in this first sample were heliozoans of the
species Actinosphaerium
eichornii. Finding them was lucky because its morphology is extremely
interesting, as demonstrated by the attached photos. I include the live
organism for a comparison of the information given by both methods.
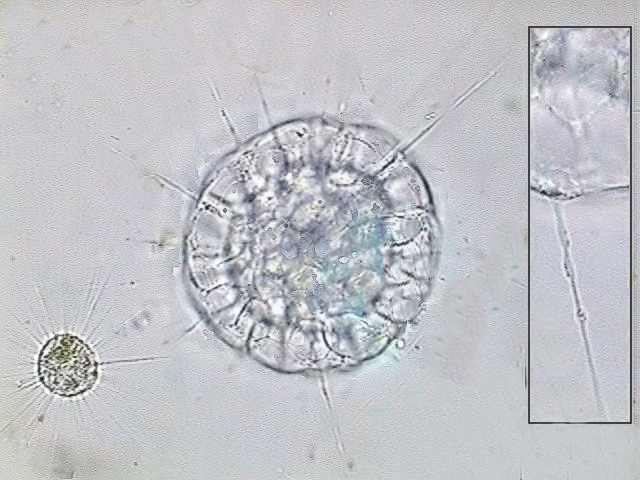
|
 |
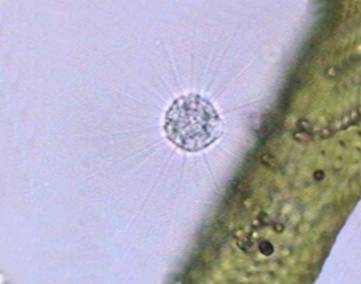
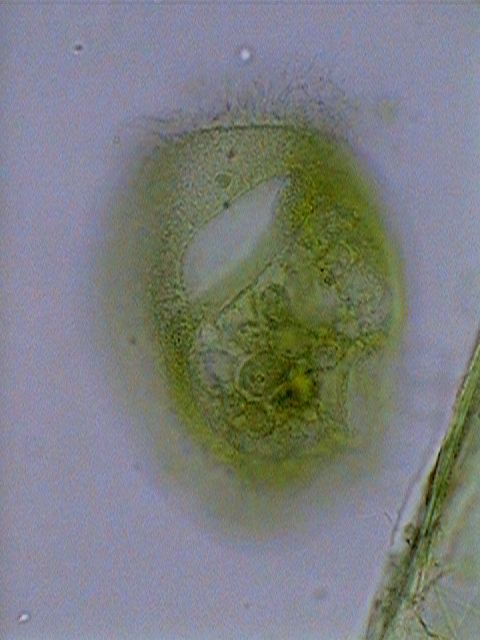
Actinosphaerium
eichhornii the larger cell, and Actinophrys sol, both alive,
to compare their sizes. The insert at right shows one axopod of A. eichhornii,
with the characteristic beads of cytoplasmic flow over the rigid
axis.
|
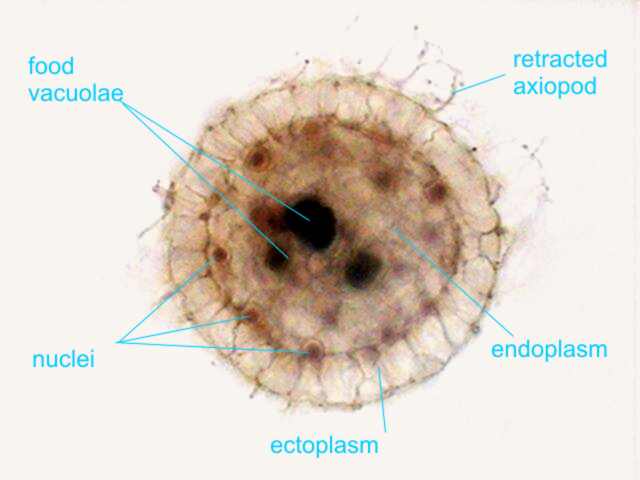
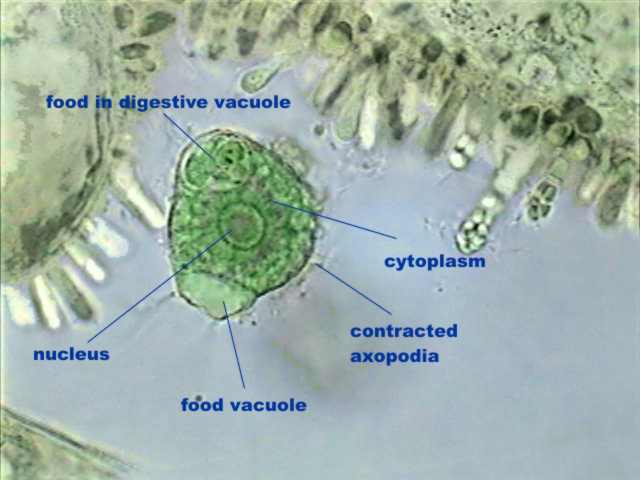
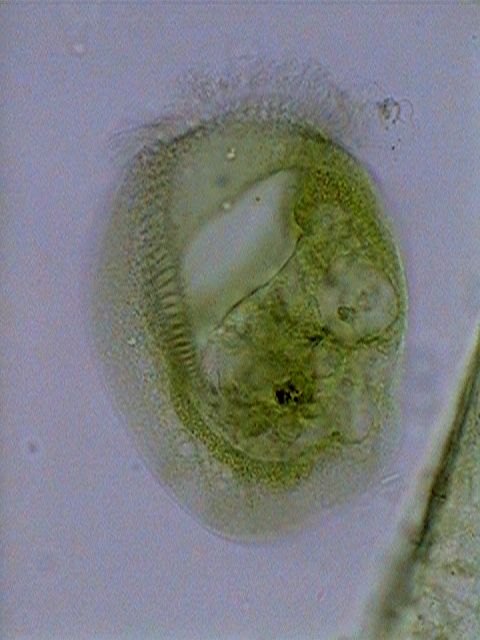
Actinosphaerium
eichhornii, stained with Allura Red, which displays its
cellular structures, including the multiple nuclei.
|
Pictures of
other organisms were not taken then, but I believe that the onion
epithelium presented in my article on the onion skin, which is still
in the same condition, and the ones of Actinosphaerium,
are sufficient to think
that it would behave more or less like the Fast Green, which I discuss
next.
FAST GREEN FCF
There are
three other water soluble dyes; sold in Mexico and the U.S.A. for dietetic aims (other manufacturers
sell similar products in the United Kingdom and the European Union).
I prepared a sample with the
same method using Green
#3. This was a dye very
well known
and used in professional microscopy: the Fast Green FCF (see
the Appendix
note on the probable present composition of the green dye).
I had been lucky
because this time (a month after the first sample), the alga was
luxuriant and the
population very diverse.
The
following pictures are a small gallery of the organisms found. Of
those the most interesting one is Euplotes (that also prompted
a special article)
which not only shows very good
anatomical details, but even allows differentiation of the macro and micronucleus characteristic of the
species. Pictures of this organism are gathered in a special table at the end
of this section.
The other
species with enormous representation in the sample was Actinophrys sol.
Of course
in the case of both heliozoans, the axopodia were fixed in different
states of
retraction. But no professional method can do better than that. There
is a clear
differentiation between ecto and endoplasm, and the nuclei and
digestive
vacuoles are clearly visible in both species.
|
|
|
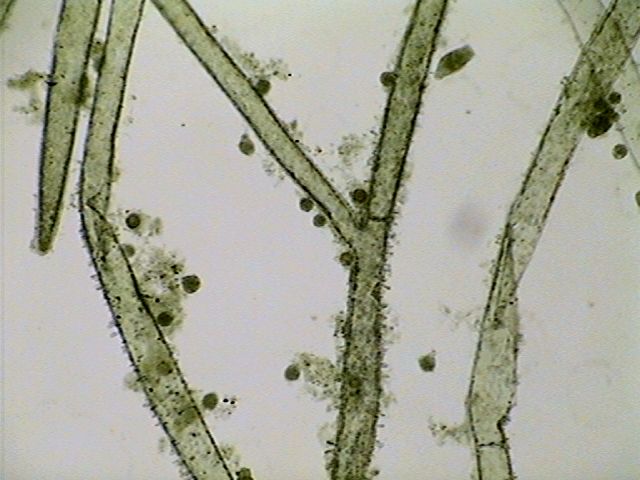
An x 4 obj.
view of some branches of the thallus, showing epiphytes and “neighbors”
mostly Actinophrys.
|
One live Actinophrys,
displaying its normal relationship with the algae. This is its hunting
position.
|
|
|
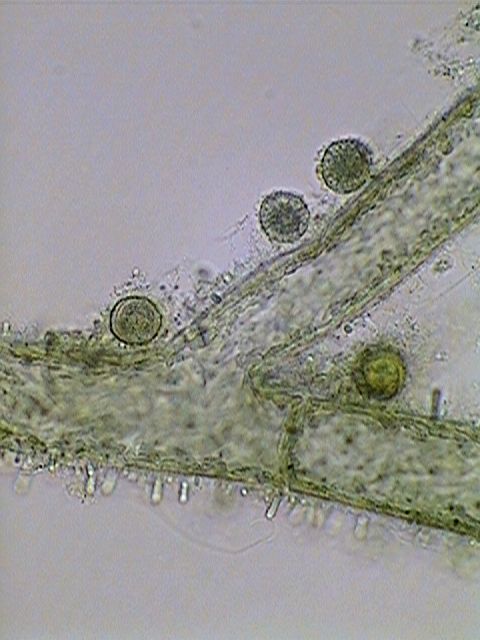
The fixed Actinophrys.
Also seen in the pictures are many epiphytic cyanophyta.
|
|
|
With the
100x objective, the cell anatomy is shown, you also see the epiphytic
cyanophyta, which will be discussed in another article.
|
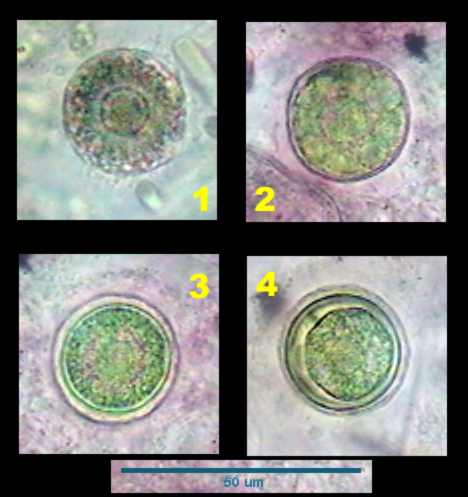
|
Four steps in the
development of a cyst. Probably a reproductive one.
|
A Stentor
population
was also extremely abundant. But the rough treatment applied was not
the best
one to fix them in good conditions of conservation. Nevertheless it is
easy to
discover the striated pellicle, as well as the peristomal ring and the
cytostome
and gullet, although the opacity of the voluminous cytoplasm hides the details of the long beaded nucleus.
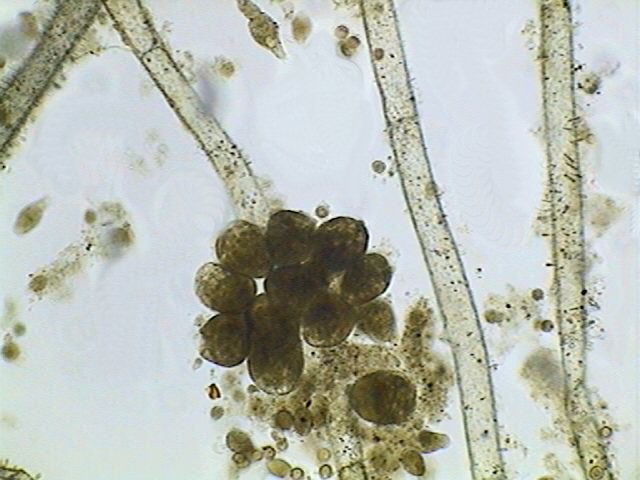
|
A
cluster of contracted Stentors fixed to a Cladophora thallus.
|
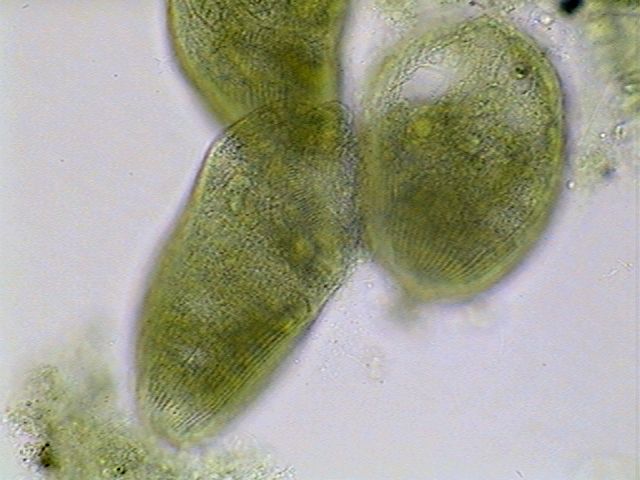
|
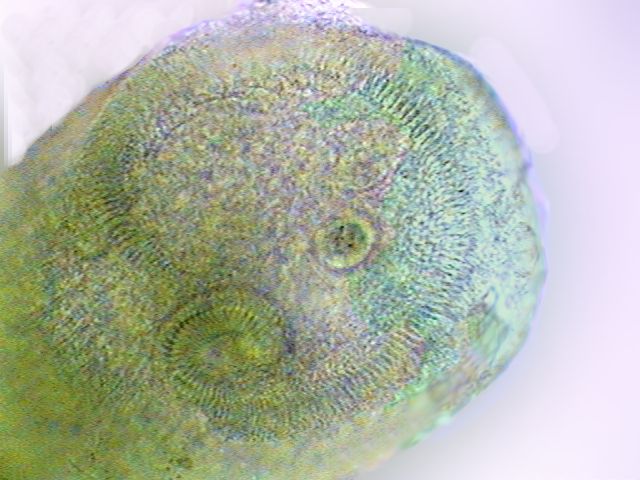
|
|
Three
stentors showing pellicle striation.
|
The ciliated
crown of membranelles around the peristomal field and the spiral
cytostome of
another stentor individual.
|
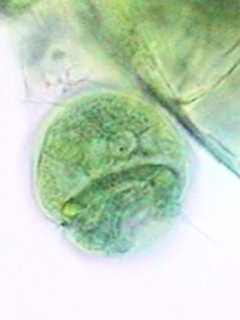
A Carchesium (Ciliatea,
Peritrichida,)
was also abundant. With shame I share a very bad picture of the little
arbuscle
and one contracted individual showing the characteristic horse-shoe shaped
nucleus.
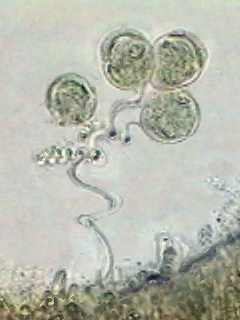
|
|
|
Carchesium
(all the zooids are contracting independently) stopped in its
contraction by
the hot fixative.
|
A Carchesium
zooid showing the well stained horse-shoe shape nucleus
|
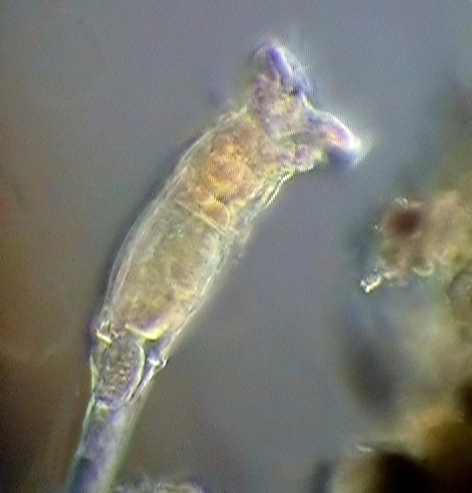
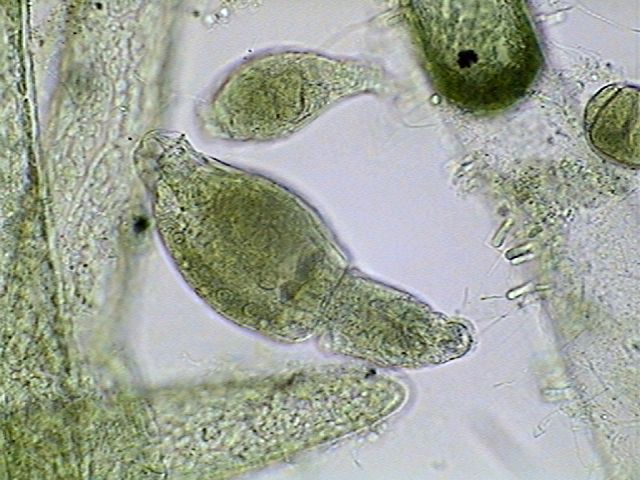
Of course
they were Bdelloids distributed throughout the preparation, but we know
very well
that these rotifers hardly lend themselves to being fixed in extension.
Nevertheless the following figure shows that the internal anatomy,
including
the germovitellarium, are well preserved and
differentiated, telling us that many Monogononta,
that could respond to anesthesia, could probably be fruitfully colored.
|
|
|
Philodina
alive,
taken through a COL-D3 filter
|
Philodina
semi-contracted x 40 Obj.
|
Numerous
eggs in many stages of development were adhered to filaments of the
alga.
To add a
different example of the uses I found for my green dye, these are
two
samples of
pollen,
colored in Fast Green FCF
and mounted in PVA-G. Of
course the onion
skin also
behaves very well with it.
|
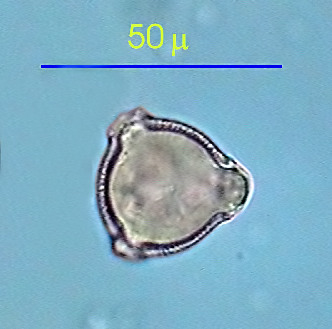 |
|
Pollen
grains of
Ipomoea colored with Fast Green and mounted in PVA-G, Obj. x 100.
|
Pollen of
Petunia grandiflora, idem.
|
NOTE:
The new web page of McCormick, the manufacturer of the dyes
that I tried, declares the green one as a mixture of Yellow #5
(tartrazine) and
Blue #1 (Blue brilliant). Apparently the Food & Drug Administration
of the EU decided
finally to prohibit the dietetic use of the Fast Green. I have not
tried new
samples and do not know what their behavior is. The sample that I still
have and
use, was declared at the time to be Fast Green FCF.
|
|
|
Euplotes
euristomum. Two pictures selected from 10 focus levels showing the
good differentiation
of the long ribbon nucleus, and the little round micronucleus
(especially in
the first picture) under the frontal border.
|
BLUE
BRILLIANT and TARTRAZINE
The other
two dyes are the blue brilliant
and the tartrazine.
Both were
proven in different and smaller samples, fixed with Gala 20, and
mounted in Glycerin, with more or less promising results.
The
following images are one picture of a gastrotrich and one of paramecium,
showing their structures stained with the blue brilliant. It must be tried
more
extensively. It is a promising dye.
|
|
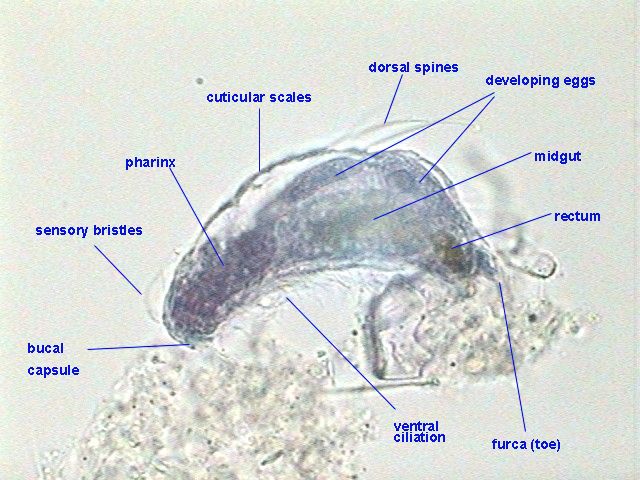
Chaetonotus.
A gastrotrich, Gala 20, Blue brilliant, mounted in glycerin
|
|
|
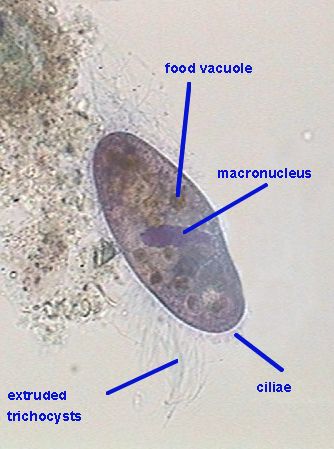
Paramecium,
from the same slide
|
The tartrazine is much
less useful because it stains in a diffuse manner,
hardly
differentiating the nucleus, or other cytoplasmic structures.
|

|
|
|
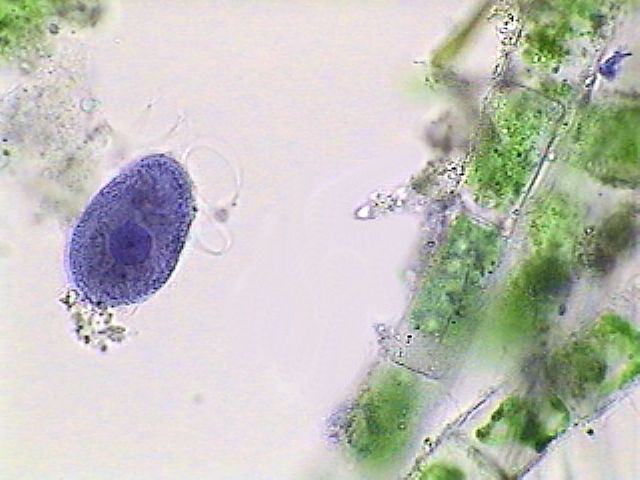
A ciliate,
stained with Blue Brilliant, in another similar preparation
|
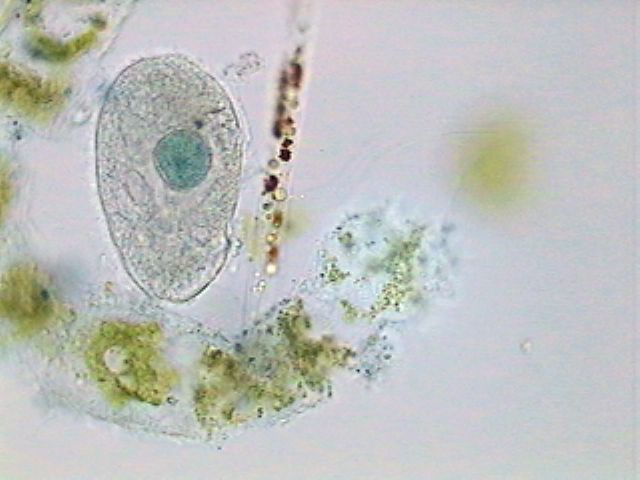
Paramecium,
stained with tartrazine
|
A little
ciliate (x 100 obj.) stained with malachite green. But it took more
than 8 hours
to reach that level of staining. I do not recommend it (at least with
these
techniques)
|
Although I
think that tartrazine
can be used with advantage to color in diffuse but intense
form the
micro-invertebrates in samples in which they must be counted. Small
micro-invertebrates
especially protozoa, gastrotrichs and rotifers are many times hidden by
fine
sediments in the sample. To color them aids searches and counting. For
this
function a stain very much used was Rose
Bengal, extremely expensive and now discarded as extremely
dangerous. The tartrazine
must be proven as an alternative. It is
fast, it
colors cytoplasm fundamentally and not detritus, it is very visible.
These are
all basic features for bulk sample coloring.
CONCLUSIONS
Four
dietetic dyes, economical and easily found in any supermarket, can render
useful
services to the amateur. Not all microscopists, especially the young
ones, can
reach, by their price or its availability, the professional dyes.
All amateur
microscopists are used to working with methylene blue, which is
sold for aquarium use; eosin,
which is
sold in pharmacies like a disinfectant, (see JMC article for reference)
and gentian violet,
(see my April 2004 article)
also obtainable in
pharmacies like a therapeutic for pathogen yeast infections. Adding to
them the very
well known Lugol’s Iodine
(or the Rhode's
fixative, see my September 2003 article),
and Chinese (or Indian) Ink,
that can be
used
for example like a
substitute for Nigrosine,
in the so called "negative"
coloration of ciliates (see
Deflandre), we
reached the number of nine available dyes for beginner microscopists, that
can
cover an ample range of techniques.
There are
other dietetic dyes and lakes that must be tried, and other sources of
colorants (e.g. the textile dyes). I hope that this article
stimulates the
search for the useful ones, and that those who attain some good
results, have
the kindness to share these with other amateur microscopists.
REFERENCES
W. Dioni, 'About microscopy and the chemistry of nail polish' Micscape August 2002
The
McCormick page
http://www.mccormick.com/productdetail.cfm?id=6036
Jean
Marie Cavanihac, www.microscopies.com
G.
Deflandre:
XXX Microscopie Practique. Lechevalier, 1947, 430 pgs.
The Stain
File, http://home.primus.com.au/royellis/stains.html
Histology
Stains:
http://www.laddresearch.com/General_Catalog/Chapter_2/LMStains/lmstains.html
W. Dioni, 'Drawing your microscopic subjects. Euplotes euristomum ...' Micscape Sept. 2002
W. Dioni, 'No formalin, no mercury fixatives. Part 2' Micscape Sept. 2003
W. Dioni, 'A cheap and precise slicer for teaching botany' Micscape April 2004
APPENDIX
European
sources for professional dyes
Brunel Microscopes
Marcel Lecomte:
http://users.skynet.be/Champignons_passion/
| The most
usual
dietectic dyes |
name
|
FD&C
|
EU
|
Tartrazine
Sunset Yellow
Erithrosine
Allura Red
Brilliant Blue
Indigotine
Fast
Green FCF
|
Yellow
#5
Yellow #6
Red #3
Red #40
Blue
#1
Blue
#2
Green
#3
|
E 102
E 103
E 127
E 129
E 133
E 132
INS143
|
|