| THE RIGHT
STUFF
The bottles that I've found are white
plastic, 75 cc, containing acetaminophen, aspirin, vitamins, or various
herbal supplements. Look for "75 cc" in small raised letters on the bottom
of the bottle.
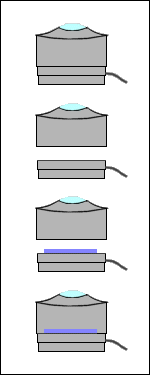
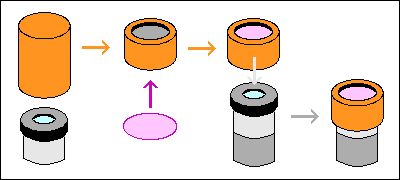
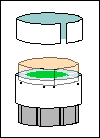
Check the fit of the piece of bottle and
your illuminator lens housing as soon as you've cut it. You may have to
shave the cut edge of the bottle to make it fit the lens housing perfectly.
If your bottle needs more work than that, heat it in a container of water
in a microwave oven and, with your winter gloves on, stretch it while it's
still hot. Do not despair— you're dealing with microns of thickness
here.
To find the odds and ends I mention,
just keep your eyes and mind open at home and in stores. Try yard sales,
"dollar stores," surplus hardware stores, etc. Richard Howey told
me about American Science & Surplus, a wonderful source of much that
others would call junk; this link will take you to the
optics section of their on-line catalog. You may find some items
too costly (e.g., 75¢ more than the local "dollar store"), but you
may not find inexpensive polarizing scraps or "redundant reticles" elsewhere.
Low-Cost/No-Cost
Stops and Filters can be made from
unlikely stuff. It's very nice to have darkfield- and Rheinberg-background
stops made of 2-mm (or even 1-mm) thick glass, but thinner transparent
(even translucent) plastic will work.
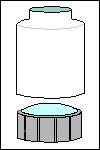
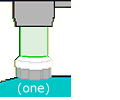
For the 30-mm
transparent disk that fits a standard filter holder, look at the stiff,
transparent packaging that came with your recent purchase of anything retailers
want to keep out of shoplifters' pockets: an ink cartridge for your printer,
a dimmer-switch for your ceiling light, electronic components, etc. Find
some that's at least .25 mm thick; cut out the flat parts with a safety
knife, and then cut out flat 30-mm circles with a scissors.
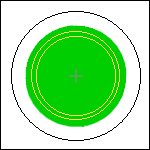
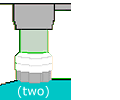
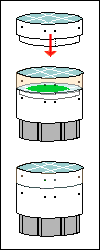
Find a plastic
bag that a loaf of bread came in, or some other soft plastic with areas
of color that are not truly opaque (true opacity produces a black background).
Cut out a 12-mm circle of each color you want to try as the background.
Center this circle on a 30-mm disk, cover that with another transparent
disk, put it in your filter holder, and look at something with your 10×
objective. Repeat for as many colors as you want.
(If you tend toward
perfectionism, you really should see how small you can make the colored
circle and still get a dark-field effect; this is particularly relevant
to stops for higher-power objectives.)
Having decided
which colors you want as background, make a 24-mm circle to try with your
higher-power objective(s). Reduce its size to what's still workable, and
make your other larger circles of colored material.
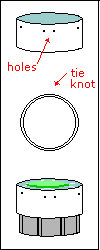
Time to decide:
do you want to have a finished Rheinberg-illumination stop for each color
you like? if yes, do you want the colored stop glued to a disk and let
be? or do you want to sandwich the colored piece between two transparent
disks? or do you want to leave your colored stops loose and place them
between transparent disks each time you want a specific effect?
Don't try to make
a whole set of these all at once. Make one and enjoy using it before spending
more observation-time on filter fabrication. |