|
SAFE
MOUNTING
MEDIA FOR MICROSCOPY TEN YEARS AFTER
|
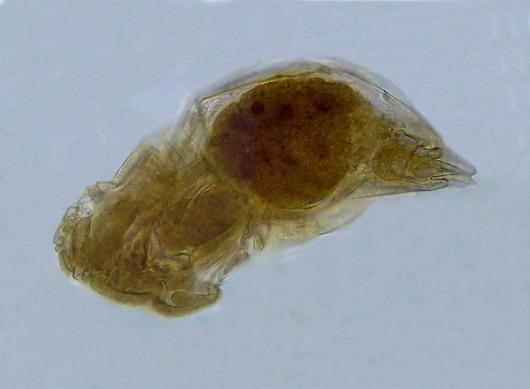
A rotifer from a shallow pond near Durango,
Dgo., México, mounted in glycerin 10 years
ago
JUSTIFICATION
Alexandre Dumas was the author of the famous book "The Three Musketeers". In his time of course they had no cinemas, nor movies, but they already used "sequels".
"The Three Musketeers" had its own sequel, which was called "Twenty Years After".**
**He even wrote a sequel for the sequel "The Viscount of Bragelone".
When I wrote the articles on the mounting media (2002 - 2003), especially the last one, I did not think at all of writing a sequel. No thought at the time, nor do I think now, to make a review of my preparations "Twenty Years Later."
However I believe that as it's now ten years since I
started my first experiments, in early 1999,
on healthy mounting media, safe for amateur
use (or whoever wishes to try), is a good
time to attempt a review.
SLIDES OR PICTURES?
Amateur microscopy has developed a lot since I found the first articles published on the Internet. It has increased the number of groups, and, especially, the used equipment offered has become decidedly at a quasi professional level; with a profusion of famous microscope brands, and quantity of Phase Contrast or Difference Interference Contrast equipment. A researcher, a rotifer specialist, high-level, and with numerous scientific publications, told me that he does not have at his disposal the high quality equipment which the more advanced amateurs possess..
Along with the microscope, the essential equipment of any amateur at any level, photomicrography has developed, driven primarily by the availability of digital cameras (a dream no one dreamed of 30 years ago) at all levels of quality and price, many of them more or less easily coupled to a microscope.
The computer is now natural equipment for a student, and along with them the "webcams”, currently essential auxiliary equipment, are purchased, which have now reached a resolution of 2 Mpx, with video capture speeds of 60 fps. Digital cameras have become common, with a quality at an unimaginable level 5 years ago, which now offer 12 and more Mpx resolutions. The range of prices and quality is so broad, and new developments so frequent, that it has become customary to consider disposable the “old” cameras. And with any of them, any microscopist, with a little experimentation, can access photomicrography.
Living organisms, their morphology, color, and behavior can thus be well documented. And for an amateur this (now common, and previously impossible ability) generally meets all their needs. Therefore, the pictures, and video files, replaced to a large extent the slides, (difficult to prepare, maintain and store) that were the previous target of amateurs, as evidenced by the valuable review articles on old "slide-makers" regularly published at MICSCAPE, and such preparations are used to illustrate articles and discussions of microscopists.
However, even with the help of the frame-stacking programs to recover the field depth (e.g. CombineZ) it is impossible to capture in a single exposure all the details of an organism. In my work on the Bdelloid Rotifers I left a list of the details (and therefore images) needed by someone seriously interested in achieving the ability to determine the species. The more advanced amateur, seriously interested in the scientific discipline that he explores, will often want to re-examine some specimen, because of a desire to learn more about this species, or to use a newly acquired key, or for comparison with a new-found specimen, or to share their findings with a specialist.
Even if micrographs can be a goal in themselves, by their technical quality, or their beauty (there are forums to display them, and share them, and contests especially dedicated to promote them), the preparation of specimen slides, as a demonstration of technical skill, aesthetic satisfaction (see the incredible artistic images of old and new diatoms preparations) and also of scientific record, may also be a target for many microscopists.

Fig 1
and 2 - two designs with diatoms. Dominique
Prades pictures and preparations
(see
scopimages at
free.fr
)
If you wish to see some very old arrangements,
visit also
http://www.victorianmicroscopeslides.com/slides.htm
So I think that it's not trivial to show what happened to the slides I made 10 years ago to test the behavior of different mounting media.
For those that want to try mounting something for the first time, I think that a careful reading of the Richard Howey article Permanent Slides: Pros and Cons is mandatory. There are many examples of subjects easy to manipulate and mount. Try them also with this different mounting media.
http://www.microscopy-uk.org.uk/mag/artmar99/rhslide.html
The aim of the published series of articles was to eliminate health risks, and collect a series of formulae, useful over an ample range of different subjects, that use ingredients that were not banned or severely restricted.
Leaving aside the "wet mounts" in an
antiseptic solution (usually the same sample
water, with the added fixing agent) which,
even sealed, can only be expected to survive a few
weeks or a couple of months at most,
of the 19 mounting media tested between
1999 and 2002 those below were discussed in the last
article of the series, as candidates for
intermediate or relatively long duration (5 or
10 years) which I think, could be the objective
of an amateur microscopist.
|
Aqueous
GP
– pure glycerin |
Resinous GUM DAMAR
NPM
– nail polish enamel
Additional
|
A
reading of the original articles is necessary
for the understanding and use of this review.
For each discussed media the address of the
reference article is given under the title, go
there and return to these notes.
Pictures included here don’t claim contest
quality, they are only added as witnesses of
the formulae behavior after ten years in their
boxes. If not otherwise stated, they were
taken with the Logitech 9000. All were trimmed
and/or reduced for to present them in an
uniform size and shape.
PURE GLYCERIN (PG)
www.microscopy-uk.org.uk/mag/artdec02/wdmount2.html
This medium needs nothing more than the confirmation of its outstanding qualities. As I said, presenting it eight years ago, in museums all over the world there are all kinds of preparations mounted in glycerin for decades. The only contraindication is that it is a liquid medium, and hygroscopic, and therefore it is essential, but not easy even for professionals, to perform a very careful sealing of the preparations.
The range of subjects which can be mounted in Glycerin is extremely wide. It is interesting to note that professional researchers of nematodes and rotifers mounted them for study, and for the file of the types of new species, preferably in this medium. I found that preparations of periphyton and freshwater plankton mounted in 50-70% glycerin, have, 10 years later, the same quality, including almost the same colors they had when mounted. No dyes were used whatsoever. Preparations were ringed with nail polish. They were permanently kept horizontally.
Periphyton, washed and concentrated from
aquatic vegetation of a shallow pond near
Durango City, Dgo.
México. Fixed with
boiling water. Post-fixation with Lactocupric,
washed with clear water 2 times, and glycerin
added in approx. 5 stages, to reach 50%. Drops
mounted, slightly compressed, and sealed with
1 layer of NPM, plus a thick another one, 1
week later.
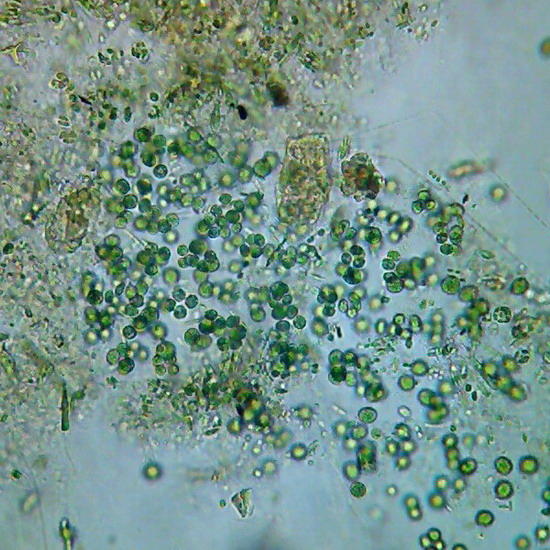
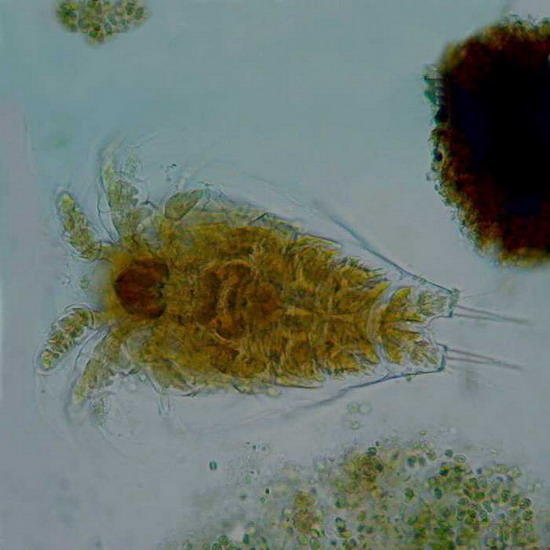
Fig. 3
- Chlorophyceae. 40x
objective,
fig 4 - A copepod nauplius, idem
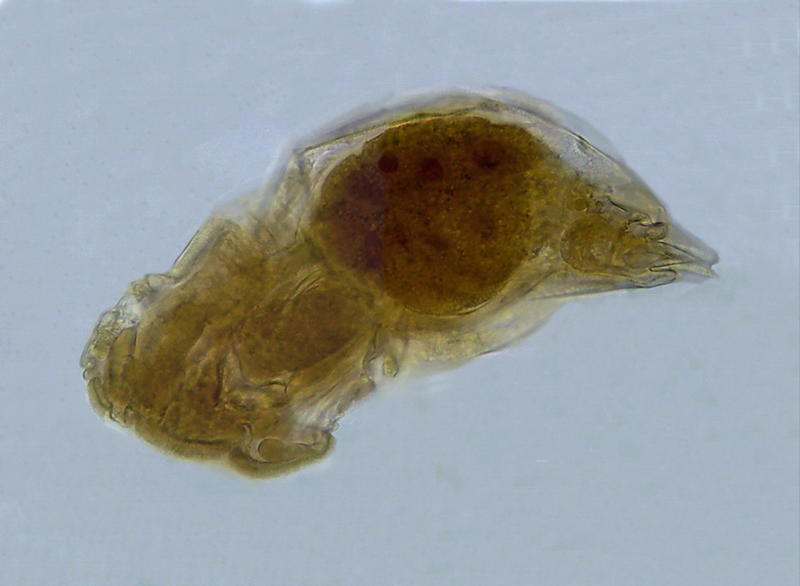
Fig. 5 – A Monogononta rotifer. 40x objective. Its opacity is just slightly more than when it was mounted.
GLYCERINE JELLY (GJ)
www.microscopy-uk.org.uk/mag/artaug03/wdpart4.html
This media is the answer (developed in the
19th century) to the problem of the liquid
state of glycerin. The addition of
gelatin allowed working more easily and
safely, by providing a solid medium.
It is another mounting medium widely accepted, and, probably, the most recommended for amateur microscopists. And widely used by botanists, mycologists, phycologists, entomologists, and histologists. The best known formula is undoubtedly the one of Kaiser (1880), which is extremely useful even in the tropics, if preparations are kept in the dark, cool, and ventilated. The ingredients are very easy to get and combine. In addition to mine, there are on the network many instructions to prepare, and to use it.
Interestingly, this formula has been a means of identifying that MICSCAPE is being used not only by amateur microscopists, but by professionals also. The formula given by me in 2003 was published in 2006 by the Univ. of Minnesota (without declaring the source of it) in their formulary. (http://bipl.umn.edu/files/Mount.pdf) The origin is easily identifiable. I modified the formula of Kaiser in order to adapt it to the gelatin source at my disposal (Knox powdered gelatin) that is sold in bags of 7 g, and to the humidity and temperature in my lab in Durango, Mexico. In addition to that, until I published my formulas, the disinfectant that was incorporated in Kaiser was phenol (now banned for amateurs) I changed it to LISTERINE. And the preparation instructions provided are practically the same as I gave. It is gratifying that MICSCAPE, while not being a "magazine controlled by reviewers", can be a source of useful knowledge at the professional level. But not always easy to verify the transfer.
Of course Glycerin Jelly is used molten, and one of the most elegant tools to work with it without trouble, as you can work with any other liquid medium, and having long enough time even for a micro-dissection, without risking adding more air bubbles that is unavoidable, is the efficient heater of J.M. Cavanihac.
http://beyond.prodelos.com/2001/sep/cavanihac/heater.html
Also for gelatins, the suitable range of
subjects is enormous. It should be
remembered that it is a good precaution to
embed the subject in glycerin, before mounting
it. There is no need to take it to pure
glycerin, a concentration of 50% is
satisfactory.
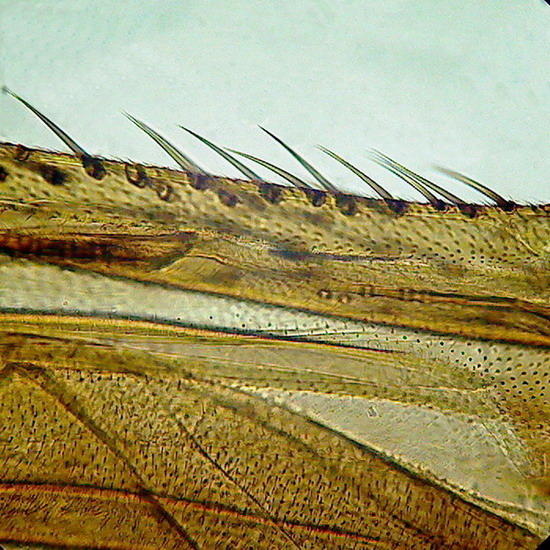
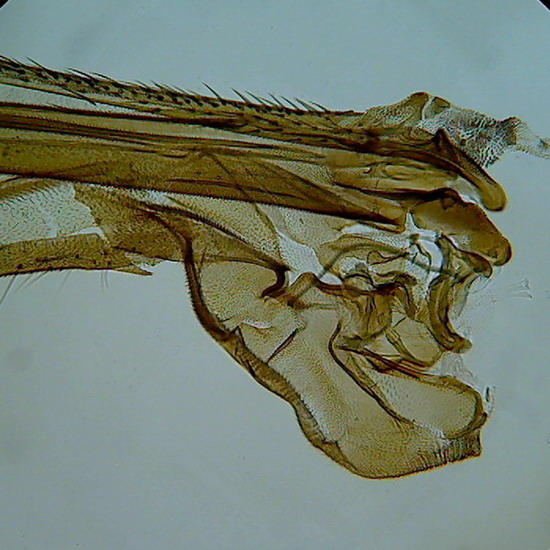
Figure 6 - Wing of a crane fly, as the borders were not sealed, and no spacers were used, the compression of the coverslip as the medium dehydrate slowly, caused some cracks in the chitin (Fig. 7)
Of course there are many published recipes using
glycerin jelly, which usually only differ in
small percentages of the ingredients, that are
not even worth trying, as the antiseptic
used to keep it free of pollutants are generally
all banned or discouraged today for health
reasons. You can use any
antiseptic compound soluble in water and
miscible with the jelly. Clear solutions of
Benzalconium Chloride are also acceptable.
PVA-G (Polyvinyl-alcohol with glycerin)
www.microscopy-uk.org.uk/mag/artapr03/wdpart3b.html
This media is not so easy to prepare outside the U.S.A. Although I get a good consistency PVA syrup in Mexico, is not as clean as the ITOYA O'Glue I recommended in the original publication, and which is what I am using still now. From declarations of South American and European correspondents it seems that it is difficult for them to identify a reliable source for this product. Except of course for those who have relationships with a well equipped professional laboratory, the chemical powder of good quality is extremely difficult to buy, and expensive also.
It is a pity, because the optical properties and conservation are very good., dries quickly and stays clear. I still recommend to strive to find a ready made water clear transparent adhesive, with the consistency of honey or syrup, which most probably is based on PVA (even if the label doesn’t declare this) by making a tour of the stores that provide materials for schools, or offices, or crafts, or shops selling holiday ornaments and accessories. I find now that it is also appropriate to seal the preparations because some show some shrinkage of the medium around the edges of the covers. It is logical, because the PVA is in aqueous solution. The ones sealed have not had that problem.
Howard Webb (see his articles in MICSCAPE) has used this medium to mount his cladocerans. At my request he kindly informed me about the behavior of the medium in these terms:
“I am still using PVA-G (just mounted some more daphnia this evening). Looking back at old slides, they seem to have held up well in general. Most of the shrinkage occurred in the first month (no prep and too much water), and there is no noticeable deterioration of the specimens. I have not sealed them in any way (as I did with jellied glycerin).
The one thing I have noticed is a lot of condensation on the slides. I have them stored in plastic slide boxes, and it looks like there has been some evaporation and condensation of something volatile. Almost all of the slides have a mist of something on them (glycerin?). They clean up fairly easily, but definitely need a cleaning.”

Fig. 8 Cross section of a stem of Epipremnum aureum, prepared with the mesotome. The image is a mosaic of 4 pictures, objective 4x.
Fig. 9-
The right is a package of vessels in the
“stele” of the same. 40x Objective

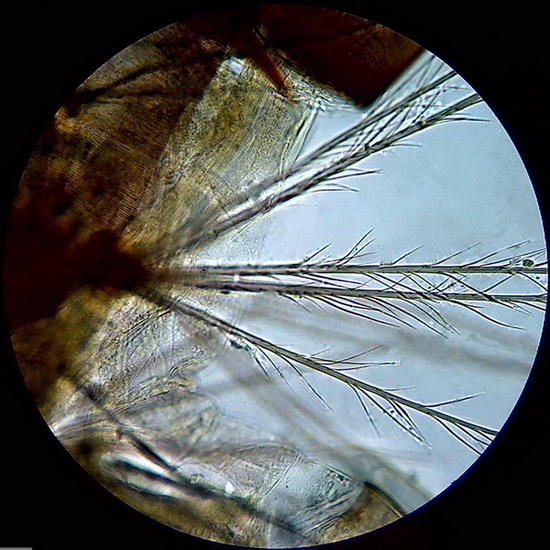
Fig. 10– on the right, topographical picture
of the caudal end of larva.
Figure 11 - On the left a bunch of spines (probably sensory) located laterally in a body segment of the larva


Gills
of a culicine mosquito, objectives 10x (Fig.
12) and 40x (fig 13) – they still show the
nuclei of cells that form the branchial
leaves. Taken with Logitech zoomed to 2.5
Mpx, more or less.
The four images are from a thick preparation, with coverslip supported by 1 mm thick supports. Larva fixed for48 hours in 70% alcohol, passed through 20% glycerol for two hours before mounting. Sealed with NPM.
Note: Unfortunately I lost the preparation of
Aptenia
epithelium mounted in PVA-G. I think it
would have performed better than the PVA-L
mounted, illustrated below.
PVA-L (Polyvinyl-alcohol with lactic acid)
www.microscopy-uk.org.uk/mag/artapr03/wdpart3b.html
There is only one Thrip preparation, photographed and published years ago on a dark background. The preparation is firm and dry, without staining of any kind, with very good transparency and cleanliness. It was not sealed. That led to more than desired evaporation of the solvent, and the coverslip compressed the insect which deformed the glass, causing a small surface crack! But there is no shrinkage at the edges. It is in all similar in quality to Gum Damar. It is worthwhile continuing to try this media , much easier to prepare than the Damar , but remember to seal to avoid this problem. I used two photos to make a collage .

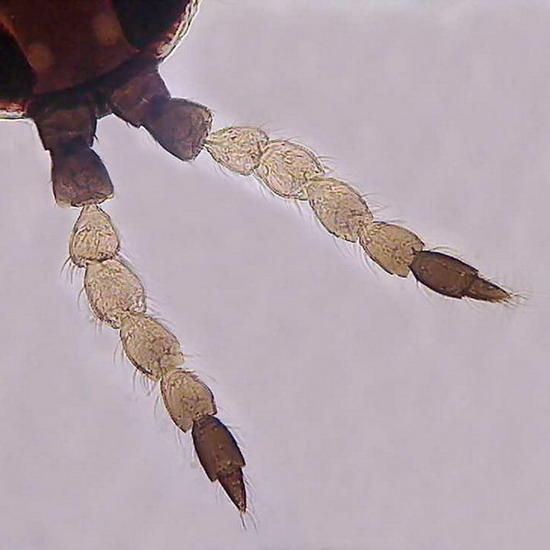
Fig. 14
Thrip, whole, obj. 4x, Fig 15 Thrip, antenae
obj. 40x
Aptenia
epithelium
was mounted without coloration, directly after
being fixed with lacto-cupric. The
epidermal peel is very well preserved, but is
practically unusable (and is all less than
photogenic) because it lost the color of the
chloroplasts, and the medium has an RI
excessive for this transparent
subject. Only a condenser central stop of
15 mm in diameter, creating a Circular Oblique
Lighting, lets me view the stomata using low
light. But this preparation shows that
the PVA-L can keep perfectly vegetable
sections. It should be checked whether it
accepts and retains colored preparations, but
its acidity is high, which could attack the
basic dyes.
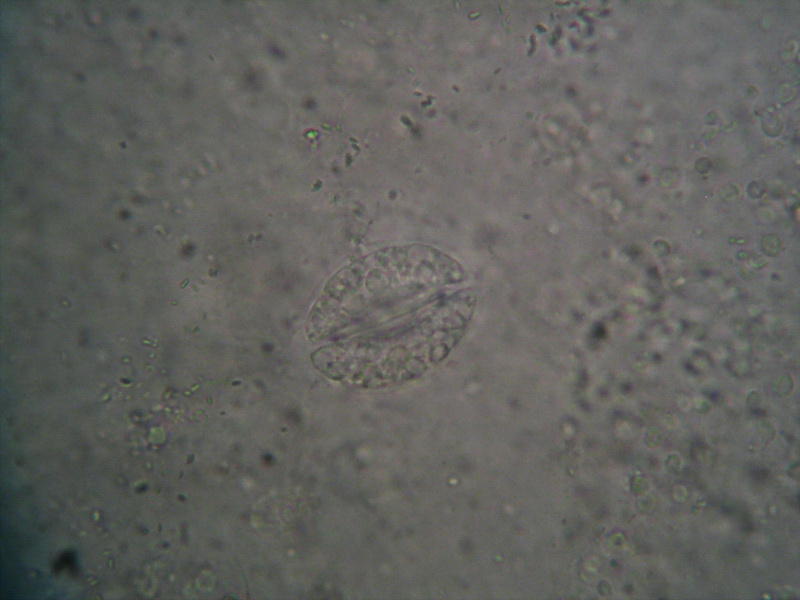
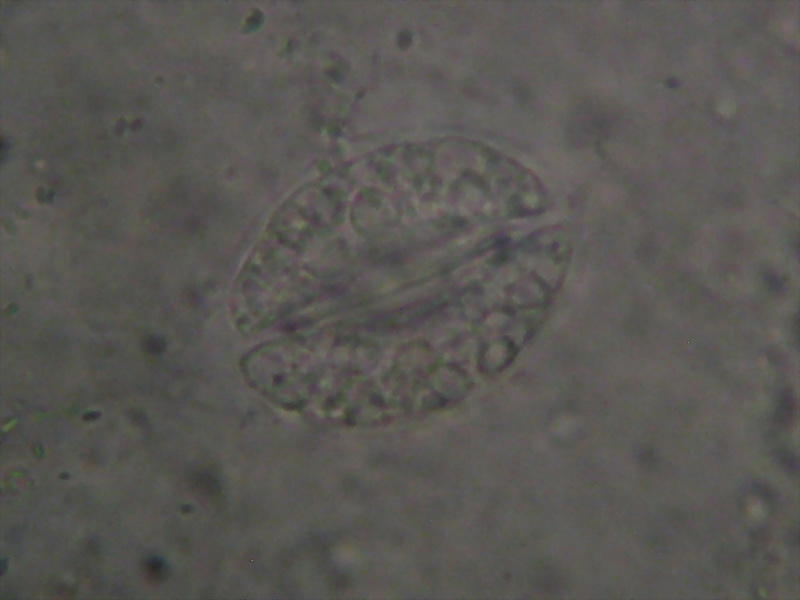
Fig.16, Fig 17
Aptenia sp. Epithelium. Fixed in Lactocupric. Mounted in PVA-L. Image using a Canon Powershot A-75 (3.2 Mpx) handheld over the objective. 100x Obj., Illumination dimmed to a minimum compatible with picture taking. Chlorophyll is totally bleached, but histology is preserved. fig 15 is the reduction of the original 3.2 Mpx picture, fig 16 is a crop of the nucleus from the original.
As in other preparations this isn't sealed. The media is completely clear, transparent and reduced to a thinnest sheet. But there is no retraction on the edges.
Not all preparations were successful.
A
mayfly
larva was mounted in PVA-L and at the time
it allowed great pictures in Dark Field and
Rheinberg. It is now completely broken down
and surrounded by an elliptical gas bubble
that was rejecting the mounting medium
around the larva, carving irregular
channels and islands of varied relief. My
hypothesis is that the larva, although I have
recorded that was fixed in alcohol 70 was not
fixed (ooho! That’s shameful!)... or poorly
fixed. I think the generated
decomposition gases slowly carved the
bubble. This means that failure is a
fault of the technique, and not of the
mounting medium. No retraction at the
borders.
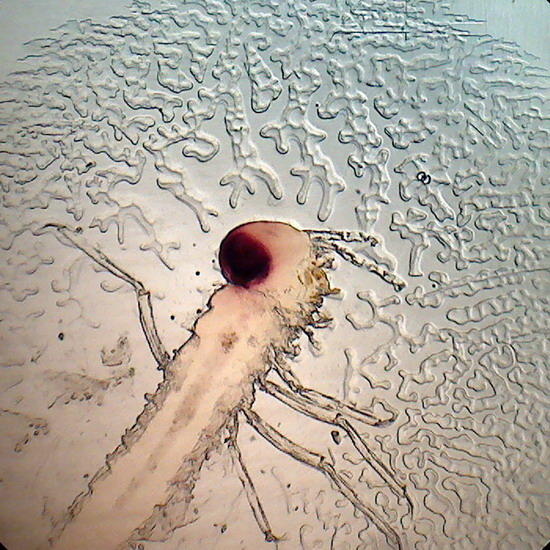
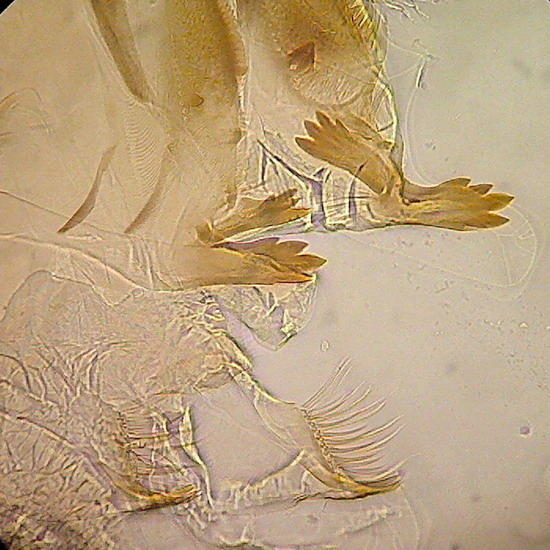
Fig 18 - general view Fig 19 - Mouthparts of the mayfly larvae.
Tangential
cut from a Geranium stem. Mounted to
observe the spiral vessels, and calcium
oxalate druses. Fixed in AFA, without
coloration. The vessels look good, as
well as crystals. The medium is clear,
transparent, dried too much and the coverslip
is deformed, as in the case of the
Thrip. The photo was taken with COL (15mm
stop) There is also no retraction.
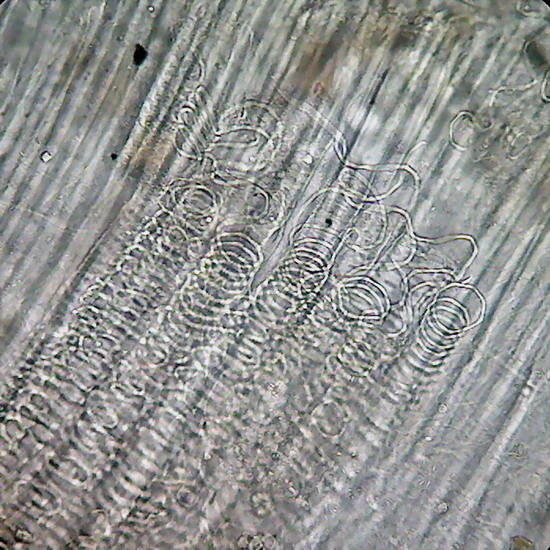
Fig
20 -
spiral
vessels of "Geranium."
Aphids. They
suffered the same bubble problem of mayfly
larvae. They do not deserve a
picture.
FRUCTOSE SYRUP and KARO
www.microscopy-uk.org.uk/mag/artjan03/wdmount3.html
It is an inexpensive and extremely useful medium. Although does not seem to be so common in Europe (and I do not know what is available in the rest of the world) KARO is a common product on the shelves of supermarkets in the United States and Latin America.
Where it doesn’t exist, crystalline, or powdered Fructose, is surely available in the dietary sweeteners section of the supermarkets. With this it's easy to prepare the syrup of Larry Legg, which is practically equivalent;
http://www.microscopy-uk.org.uk/larry/sugar4.html
Fructose Syrup is thus an absolutely safe mounting medium, easy to prepare and to use, and available to a very large audience. It is widely used by mycologists and phycologists as a preferred mounting medium.
The chloroplasts of Aptenia leaves epithelium,
fixed with lactocupric, retained their green
color. Karo also retained the Gentian Violet I
applied to onion epithelia. And copepods
mounts are among the best I have.
The image of a copepod, used to illustrate Rheinberg illumination in the 3rd part of the articles on the Logitech webcam, is from a Karo mounted female.
I think that its utility, availability and even a good refractive index (1.48) recommends Fructose Syrups, handmade or commercial, as a general mounting media for amateurs. The only problem, not a minor problem for novices, is that it exerts a heavy osmotic pressure, similar to glycerin. And for delicate materials also needs a step by step mounting protocol.
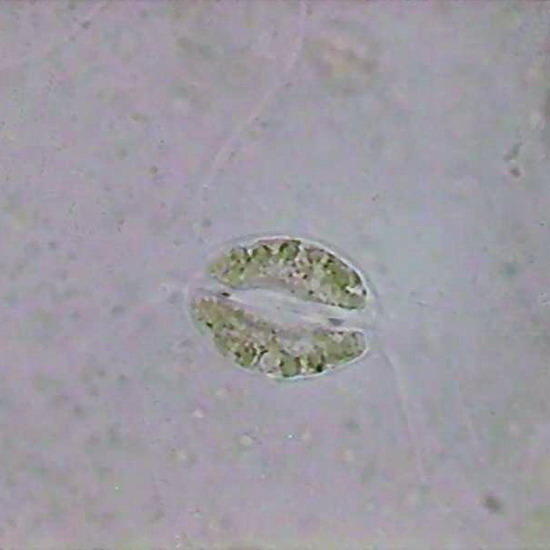
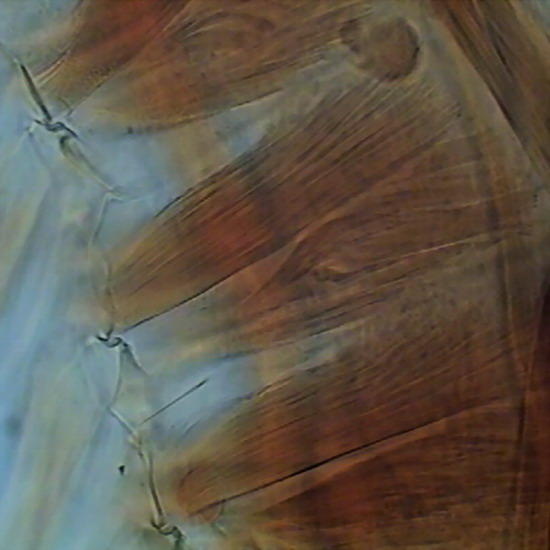
Fig. 21 Aptenia epithelium, a stoma with still coloured chloroplasts (100x – Lactocupric Fixative. – Karo
Fig. 22 Muscles that move the swimming legs
coxae of a copepod
– Lactocupric
Fixative – Karo
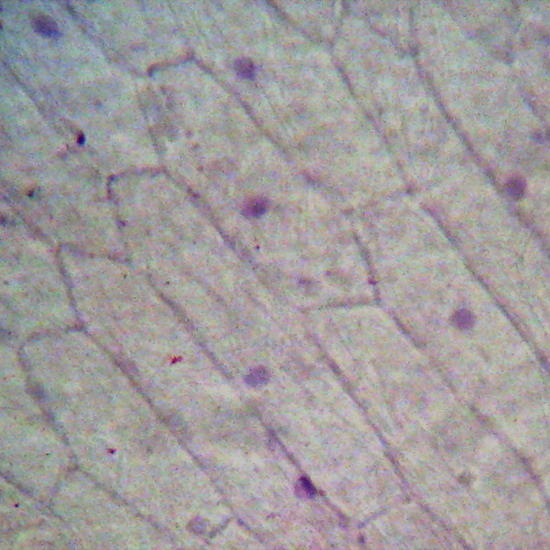
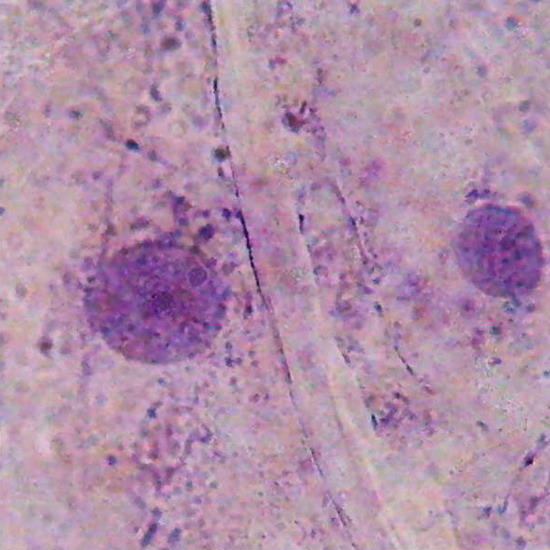
Fig. 23 Onion, epithelial cells (10x) stained with gentian violet. Focus on surface. Karo – Fixed with AFA
Fig. 24 Nuclei of onion epithelial cells, colored with gentian violet (100x). Karo – Fixed with AFA
In the next article I will present some examples of preparations from the five mounting media which still lack a review.